Understanding Portal Vein Aneurysm and Extrahepatic Portal Obstruction
The story of portal vein aneurysm (PVA) intertwined with extrahepatic portal obstruction (EHO) is both a fascinating and cautionary tale in modern medicine. When we look at these conditions, we see not only their existence as rare vascular events but also a journey that involves tricky parts of diagnosis, the tangled issues of treatment, and the subtle progression of disease. In one representative case, an adult patient’s condition evolved from an incidentally discovered PVA into something that required careful management—a progression that reminds us to steer through each clinical challenge with patience and close monitoring.
Experts emphasize that while such hepatic vascular complications might seem off-putting, they also offer an essential opportunity to learn about the small distinctions in disease progression and treatment. Given this backdrop, today we get into the nitty-gritty of how anticoagulant-induced thrombosis can lead to collateral vessel formation and how, in some cases, what appears to be an idiopathic EHO may actually have a secondary cause from PVA thrombosis.
Treating PVA Thrombosis with Anticoagulants: A Closer Look
In the case we examine, an adult male patient first experienced vague symptoms like dull abdominal pain and fever. However, a closer look using advanced imaging techniques revealed complicated pieces in his portal vein system that set the stage for further management decisions. His earlier incidental finding of a PVA was followed by the formation of thrombosis—a process that eventually diminished with the careful application of anticoagulant therapy.
Anticoagulant treatment, such as the use of heparin followed by warfarin, played a key role in alleviating the patient’s symptoms and reducing inflammatory markers. In this case, the managing team had to find their way carefully by monitoring critical parameters like AST, ALT, and clotting factors. Adjustments were made based on daily tests—a reminder that healing and recovery often depend on a delicate balance. The twists and turns in his treatment course highlight not only the power of modern therapy but also the nerve-racking nature of adjusting dosages in real time.
Key Benefits of Conservative Therapy
When it comes to addressing the potential risks of PVA thrombosis, conservative therapy with anticoagulants has several advantages. By choosing a systematic approach:
- Physicians can reduce the threat posed by shrinkage of the thrombus.
- Patient monitoring offers a chance to catch any subtle details that could indicate further complications.
- The non-invasive nature of this approach often limits additional risks tied to surgical intervention.
This strategy, which emphasizes timely treatment and close follow-up, seems especially promising in cases where there is no immediate need for an invasive procedure. Instead, with a careful evaluation of lab results and imaging, clinicians can measure the impact of therapy on both the aneurysm and the accompanying collateral vessel formation.
The Role of Imaging Techniques in Diagnosis and Follow-Up
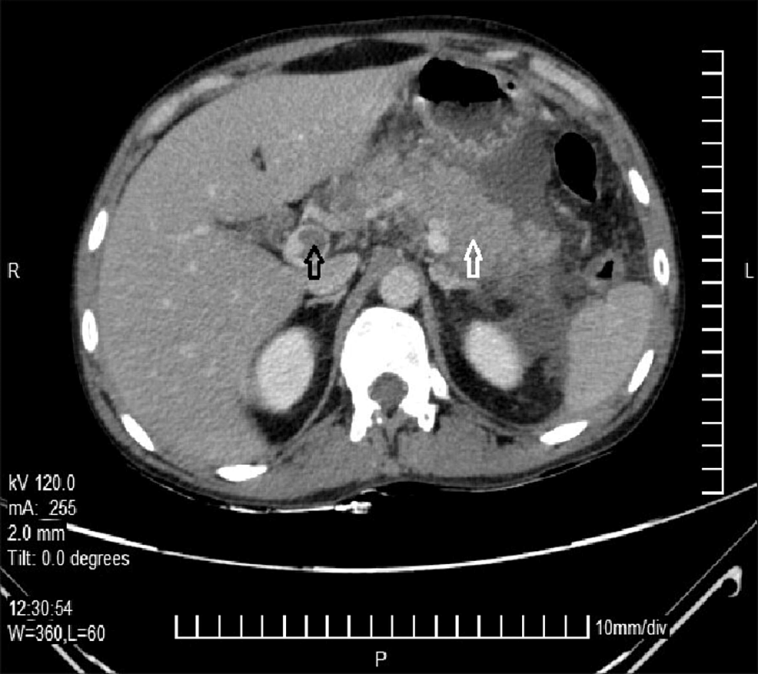
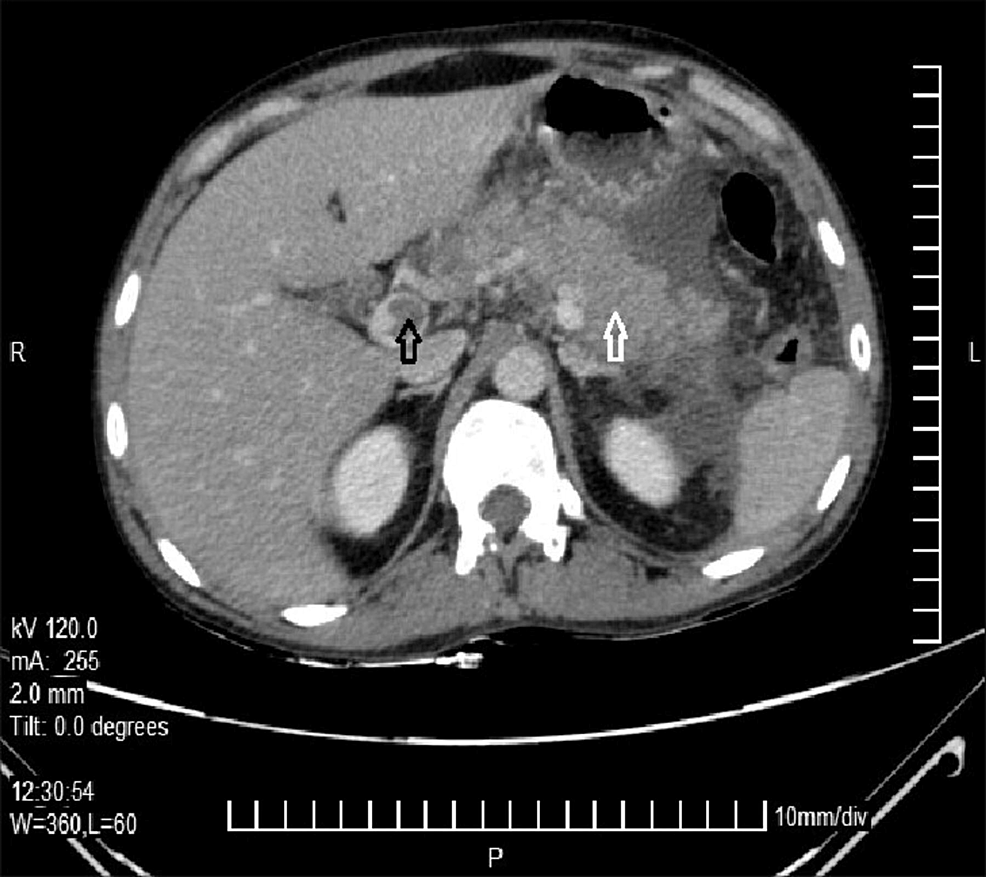
One of the most impressive aspects of modern vascular medicine is the availability of sophisticated imaging techniques. In the specific instance of PVA evolving to EHO, imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI) with gadolinium enhancement, and ultrasonography (US) revealed the fine points of the evolving pathology.
For example, a CT scan initially uncovered the existence of a PVA and measured its dimensions carefully. Later on, an MRI helped reveal both the extension of thrombosis along the portal system and even the formation of collateral blood vessels after the PVA diminished. Ultrasonography provided additional confirmation—with Doppler studies, the absence of blood flow in the main portal vein trunk was noted, while surrounding collateral vessels started exhibiting flow. These imaging techniques offered a roadmap of the patient’s journey, proving invaluable in managing his condition.
Table: Comparison of Imaging Techniques for Vascular Diagnosis
| Imaging Modality | Key Findings | Advantages | Limitations |
|---|---|---|---|
| CT Scan | Initial detection of PVA; measurement of aneurysm diameter | High resolution; quick results | Exposure to radiation; contrast material sensitivity |
| MRI with Gadolinium | Extent of thrombosis; non-enhancement areas within the vessels; collateral formation | No radiation; excellent soft tissue contrast | Longer exam times; contrast agent contraindications |
| Ultrasonography (with Doppler) | Real-time blood flow analysis; identification of collateral circulation | Bedside availability; non-invasive; cost-effective | Operator-dependent; less detailed in complex anatomy |
This table emphasizes how each imaging modality has its own set of advantages and challenges. Whether it is the quick turnaround of CT or the detailed blood flow analysis provided by ultrasonography, the choice of technique is driven by the need to manage each tricky part of the case.
Recognizing the Collateral Vessel Phenomenon
One of the most intriguing developments in the managing case was the emergence of collateral vessels after the PVA thrombosis. When the main portal vein’s blood flow becomes compromised, the body attempts to compensate by creating alternative pathways. These new channels are essential in maintaining hepatic circulation, ensuring that the liver cells receive enough oxygen and nutrients.
This phenomenon, while impressive from a physiological standpoint, can also be a source of diagnostic confusion. Clinicians must take a closer look and not jump to quick conclusions. Without ongoing imaging and laboratory tests, these events might be misdiagnosed as primary EHO—when in fact, they may be secondary to the initial PVA thrombosis. It is an example of how important it is to get into the details of the evolving vascular network rather than merely relying on a single snapshot in time.
Understanding Collateral Formation: Key Points
- Collateral vessels develop as a compensatory mechanism in response to decreased main vessel flow.
- Such vessels may mimic primary disease when viewed out of context.
- Regular follow-up imaging helps clinicians figure a path and correctly identify the origin of the obstruction.
- This phenomenon necessitates cautious and continual diagnostic review over time.
The case highlights how important it is to manage patient follow-ups. Without regular check-ups, the subtle details of collateral vessel formation could lead to a misclassification of the patient’s condition. It is crucial to recognize that the clinical process is a continuum, and that the evolution of collateral pathways is an integral part of many vascular disorders.
Troubleshooting Diagnostic Challenges in Hepatic Vascular Disorders
Accurate diagnosis of conditions like PVA and EHO is a process loaded with problems and confusing bits that require a close reexamination at every step of the patient’s journey. One of the chief challenges is distinguishing between primary and secondary EHO. At first glance, both conditions might appear similar on imaging studies, but the clinical history offers additional clues that help differentiate them.
For instance, a history of PVA detected incidentally years before the onset of thrombosis can serve as a key indicator that what may now be diagnosed as EHO might actually be secondary. This calls for a sense of skepticism when evaluating cases that seem straightforward. The possibility of misdiagnosis is always present, especially when the diagnostic timeline is not closely followed. In this context, clinicians have to figure out a path through the small distinctions and hidden complexities that separate primary from secondary EHO.
Bullet List: Diagnostic Pitfalls to Avoid
- Assuming all EHO cases in adults are idiopathic without reviewing past imaging.
- Overlooking the history of incidental PVA, which could point to a secondary cause.
- Failing to monitor collateral vessel formations in follow-up imaging tests.
- Misinterpreting laboratory markers that signal inflammatory and thrombogenic processes.
These diagnostic pitfalls underscore the delicate balance needed in interpreting medical images and lab results. Making the right diagnosis is less about ticking boxes and more about working through every subtle detail and small twist in the patient’s history and test outcomes.
Interpreting Laboratory Values: A Balancing Act
Beyond imaging, laboratory markers can be critical in providing clues to the underlying pathology occurring in hepatic vascular conditions. In the case under review, lab tests revealed elevations in hepatic enzymes and inflammatory markers such as C-reactive protein (CRP). These indicators were key in identifying the presence of thrombosis and the subsequent inflammatory reaction. Although some might perceive these findings as intimidating, they actually serve as a reassuring guide for physicians—signaling the need for timely anticoagulant therapy.
The combination of modest increases in AST and ALT with significant elevations in CRP and other fibrinolysis markers (like FDP and D-dimer) provided compelling evidence of a thrombogenic process. While the complex mix of markers might seem overwhelming, they actually offer a robust framework for determining the severity and progression of the disease.
Table: Laboratory Markers and Their Clinical Significance
| Marker | Observed Change | Clinical Interpretation |
|---|---|---|
| AST & ALT | Mild elevation | Indicates hepatic stress, likely due to impaired blood flow and thrombosis |
| C-Reactive Protein (CRP) | High elevation | Reflects an active inflammatory process; common in thrombosis |
| Fibrin/Fibrinogen Degradation Products (FDP) & D-Dimer | Significant elevation | Suggests ongoing clot breakdown and corroborates the diagnosis of thrombus formation |
| Coagulation Factors (Protein C, Protein S) | Within normal limits | Helps rule out congenital or inherited coagulopathies |
This table underscores how a nuanced interpretation of lab values not only guides therapeutic decisions but also shapes the monitoring strategies going forward. In managing these tricky parts, every number counts, and even a slight difference can mean the difference between a correct diagnosis and a misstep.
Reflections on Patient Management and Follow-Up Strategies
One of the lessons from this case is the absolute necessity of ongoing follow-up. When a patient is first diagnosed with a PVA—especially one detected incidentally—it may be tempting to consider it a static finding. However, as this case shows, conditions evolve. The absence of follow-up visits can deprive both the patient and the healthcare provider of the chance to catch the early signs of complications, such as thrombosis, before they lead to more significant vessel changes like EHO.
Follow-up not only involves repeated imaging but also a consistent review of laboratory findings. The development of collateral vessels, for instance, should be viewed as a sign of compensatory adaptation rather than a new primary disease process. In light of such systemic changes, a periodic reassessment can alert physicians to the need to adjust treatment plans—whether that involves continuing, tapering, or even halting anticoagulant therapy. This continuous cycle of review and adjustment is critical for ensuring both short-term relief and long-term health.
Bullet List: Best Practices for Follow-Up
- Schedule regular imaging sessions to monitor the size and structure of the PVA.
- Perform routine laboratory tests to track inflammation and clotting markers.
- Ensure that patient symptoms are re-evaluated with every visit.
- Educate patients about the importance of ongoing monitoring, even in the absence of overt symptoms.
- Maintain an open line of communication for any new or worsening symptoms.
These best practices are more than administrative protocols—they are a vital, hands-on approach to manage a condition that continuously evolves, requiring healthcare providers to get into the fine points of each patient’s unique journey.
Assessing the Impact of Anticoagulant Treatment on Vascular Outcomes
The use of anticoagulants in the treatment of thrombus formation within a PVA is not only a standard therapeutic maneuver but also a testament to the advances in vascular medicine. In the case under discussion, treatment was initiated with heparin, followed by a carefully modulated course of warfarin. This sequence of interventions demonstrated clear benefits: the patient’s abdominal symptoms lessened, inflammatory markers trended downward, and there was visible evidence of improved blood flow in follow-up imaging studies.
Despite the nerve-racking challenges in adjusting dosages day by day, the overall outcome was positive. The case reveals that, when handled correctly, anticoagulant therapy can shrink a thrombus and diminish an aneurysm, even if the trade-off is the eventual appearance of collateral vessels to compensate for a now-obstructed main portal vein. It is both a triumph of medical science and a reminder of the body’s intrinsic ability to adapt to challenging conditions.
Table: Anticoagulant Treatment Timeline and Key Adjustments
| Phase | Medication | Dosage Adjustments | Observations |
|---|---|---|---|
| Initial | Heparin | Started at 700 units/hour and gradually increased (up to 1,300 units/hour) | Marked improvement in abdominal symptoms and lab markers |
| Transition | Warfarin | Started on day 9 with gradual titration from 5 mg/day to 6 mg/day | Continuous decrease in thrombosis and improved collateral vessel formation |
| Tapering | Warfarin | Gradual reduction by 1 mg/day approximately every 30 days | Final discontinuation when the vascular system stabilized |
This timeline table encapsulates the careful, piece-by-piece adjustments that were essential in ensuring that the patient’s therapy was both safe and effective. By continuously reviewing treatment doses through the lens of daily tests, clinicians could manage the small twists in the patient’s condition, ultimately achieving a favorable outcome.
Expert Opinions on the Broader Implications for Hepatic Vascular Disorders
There remains a distinct debate among experts regarding the best approach for managing conditions like PVA and EHO, particularly when it comes to distinguishing a primary disorder from what could be a secondary effect of another process. Although many cases of idiopathic EHO in children are thought to be congenital, the scenario becomes muddled in adults. The adult presentation of EHO may, in many instances, be a consequence of events such as PVA thrombosis rather than an entirely separate primary condition.
Such observations call for an approach that digs into the patient’s history—looking for clues such as previously incidentally detected PVA, even if it was never followed up post-discovery. Doing so also highlights the importance of casting a wide net when investigating the cause of hepatic vascular issues. When we look into the past events, even seemingly trivial details can emerge as key indicators of what eventually evolves into a more serious condition.
Expert Commentary: Considerations When Making a Diagnosis
- Always conduct a thorough review of previous imaging data, even for incidental findings.
- Recognize that what appears as idiopathic in an adult may have later been triggered by an unnoticed event in the past.
- Understand that secondary EHO may mirror primary EHO and require a nuanced approach.
- Keep in mind that collateral vessel formation is a double-edged sword—both a compensatory mechanism and a potential source of diagnostic confusion.
- Advocate for multidisciplinary consultation to cover all the tricky parts and tangled issues in these cases.
This expert perspective reinforces the idea that the accurate diagnosis and effective management of hepatic vascular disorders require a comprehensive approach that takes into account not just the current state of the patient, but also their full medical history and the evolution of their condition over time.
Implications for Future Treatment and Patient Care
The case we are discussing not only informs current practice but also paves the way for future medical protocols in treating hepatic vascular conditions. There is a strong argument for establishing standardized guidelines that detail the best practices for follow-up, when to intervene, and how to adjust therapy for patients with incidental findings of PVA. In a field often full of problems and tricky aspects, having clear pathways can help reduce the anxiety associated with managing these conditions.
For many experts, one of the essential next steps is to develop a framework that aids in early detection and regular monitoring of PVA patients. This blueprint might include recommendations such as the following:
- Routine imaging every 6 to 12 months for patients with diagnosed PVA.
- Regular laboratory assessments to track inflammatory markers and liver enzymes.
- Clear indicators that would prompt the initiation of anticoagulant therapy.
- Structured programs for patient education to ensure they understand the significance of ongoing monitoring.
Implementing these measures could lead to earlier detection of complications and might help prevent the progression to secondary EHO. This proactive approach has the potential not only to save lives but also to reduce the nerve-racking stress associated with sudden, unmonitored clinical deteriorations.
Planning for Long-Term Patient Outcomes
To ensure that this field continues to advance, it is critical that investment is made not only in better imaging technologies and laboratory tests but also in comprehensive patient follow-up programs. By combining these elements, healthcare providers can work through the subtle parts of each case and offer a more personalized treatment plan. Ultimately, it is this commitment to detail and ongoing care that will drive improvements in long-term outcomes for patients with PVA and evolving EHO.
Closing Thoughts: A Call for Vigilance and Continued Learning
In conclusion, the evolution of a portal vein aneurysm into a thrombosis-induced extrahepatic portal obstruction underscores the need to be vigilant at every step of patient care. The case serves as a valuable reminder that even an incidental finding must be followed with caution. When a patient presents with vague symptoms like dull abdominal pain or a low-grade fever, these might be the only subtle hints that a deeper vascular process is unfolding.
It is both a challenge and an opportunity for modern medicine to find the right balance between immediate treatment and long-term monitoring. The tricky parts, tangled issues, and hidden nuances of hepatic vascular disorders demand not just technical expertise but also a healthy dose of clinical skepticism and thorough patient history review. The evolution from a seemingly benign PVA to a case with secondary EHO compels us to reexamine how we classify, treat, and follow up on vascular disorders.
For clinicians, the road ahead involves a consistent effort to figure a path through the nerve-racking twists and turns of diagnosis and treatment. For patients, this means adhering to follow-up appointments and being aware of any new symptoms, however minor they may seem. By staying vigilant and collaborating across specialties, the medical community can work toward improved outcomes in the management of complex hepatic vascular conditions.
As we push forward, it remains critical that we continuously learn from each case. Every evolution in a patient’s condition is an invitation to take a closer look at the fine details, reexamine our treatment protocols, and ultimately refine our clinical approaches. This case—rich with lessons on collateral vessel formation, the impact of anticoagulant therapy, and the imperative for long-term follow-up—illustrates that medicine is both a science and an art, where every decision must be weighed carefully in light of evolving evidence and individual patient needs.
Final Reflections and Future Directions
Looking toward the future, managing conditions like PVA and secondary EHO will continue to involve a mix of high-tech diagnostics and the traditional wisdom of the clinical practitioner. It is essential that we continue to build our knowledge base by mining through both successful cases and those that present with unexpected outcomes. Only by doing so can we hope to avoid missteps and refine our approaches in this intricate aspect of patient care.
Moreover, as new imaging modalities and laboratory markers are developed, they promise to shine a light on the previously hidden complexities of vascular conditions. Armed with better diagnostic tools and a more nuanced understanding of disease evolution, the medical community can more accurately pinpoint where the line lies between a primary disorder and a complication of another process.
Future research might focus on further elucidating the role of inflammatory markers and the body’s adaptive response in the formation of collateral vessels. Additional clinical trials and longitudinal studies could help establish clearer protocols for when and how to intervene. This ongoing inquiry is essential not only for advancing scientific understanding but also for ensuring that every patient receives the most appropriate, tailored care.
Ultimately, the case discussed here is more than a single patient’s journey—it is a call for ongoing vigilance, detailed follow-up, and a commitment to continuous learning. For healthcare providers faced with the nerve-racking challenges of evolving vascular disorders, the lesson is clear: every detail matters, every lab value is a clue, and every imaging study is a stepping stone toward a more precise diagnosis. With this mindset, we can continue to find our way through even the most tangled and intimidating scenarios, offering our patients the best possible care in the face of an ever-changing clinical landscape.
Key Takeaways for Clinical Practice
Below is a summary of the important lessons we can take away from this case:
- Incidental findings require thoughtful, ongoing monitoring and must not be ignored.
- Anticoagulant therapy, when carefully managed and adjusted, can successfully mitigate the complications of PVA thrombosis.
- Multiple imaging modalities play complementary roles in unveiling the subtle details of vascular changes.
- Regular follow-up with comprehensive laboratory and imaging assessments is essential in preventing misdiagnosis.
- Both primary and secondary causes of extrahepatic portal obstruction should be considered when evaluating an adult patient.
By integrating these takeaways into everyday practice, healthcare professionals can work through the tangled issues and tricky parts of diagnosing and managing these conditions, ensuring that patient care remains as proactive and responsive as possible.
In summary, while challenges and unexpected twists will undoubtedly continue to emerge in the realm of hepatic vascular disorders, our commitment to ongoing observation, precise diagnostics, and individualized patient care remains the most effective strategy for overcoming these problems. The case discussed here not only broadens our understanding of PVA and its potential progression to EHO but also reinforces the idea that the journey of medicine is one of continuous exploration. As we dare to take a closer look into every subtle detail, we pave the way for better, safer, and more effective treatment for all who suffer from these complex vascular conditions.
Read more about this topic at
Cavernous transformation of the portal vein
Portal Vein Thrombosis – StatPearls