Rethinking Diabetic Retinopathy: Immune Defense Versus Endothelial Mitochondrial Dysfunction
The world of diabetic retinopathy is filled with tricky parts and tangled issues that challenge physicians and researchers alike. As an editor who has watched modern medicine, alternative medicine, and nutritional approaches evolve, I find the current understanding of proliferative diabetic retinopathy (PDR) to be both fascinating and full of complex twists and turns. In this opinion piece, I will take a closer look at recent research shedding light on the distinct subtypes of PDR—one dominated by immune-mediated clearance of pathological vessels and the other by endothelial mitochondrial dysfunction. Along the way, I will discuss the implications of anti-VEGF therapy and the limitations of current animal models, as well as present alternative strategies that might transform the management of diabetic retinopathy.
The multifaceted nature of PDR demands an approach that balances clinical insight with the nitty-gritty details emerging from scientific studies. With over one in four diabetes patients at risk for diabetic retinopathy, it is essential that we figure a path through the confusing bits and fine points of retinal vascular pathology to develop more comprehensive treatment strategies.
Understanding the Distinct Flavors of PDR
Diabetic retinopathy is not a uniform disease. Recent research indicates that PDR may comprise distinctly different forms. On one hand, there are cases where the immune system appears to be actively involved in clearing pathological blood vessels. On the other, there are cases where endothelial cells exhibit significant mitochondrial dysfunction, contributing to disease progression. This dichotomy—immune-mediated vessel resolution versus endothelial metabolic stress—not only provides a clearer picture of the disease’s underlying mechanisms but also suggests that treatment strategies need to be more personalized.
Immune-Based Vessel Clearance: Digging Into the Defense Mechanism
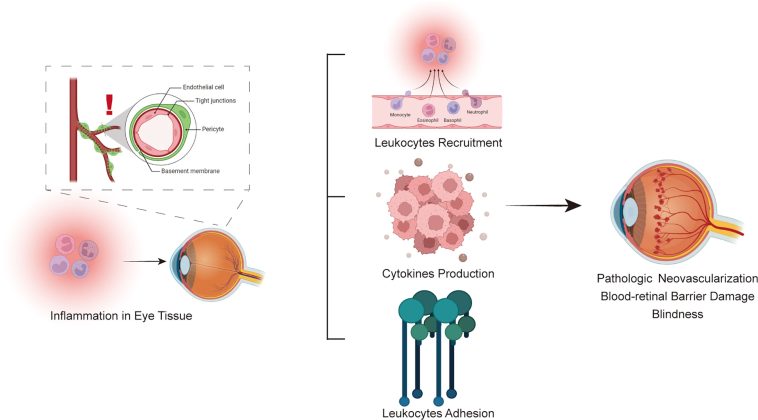
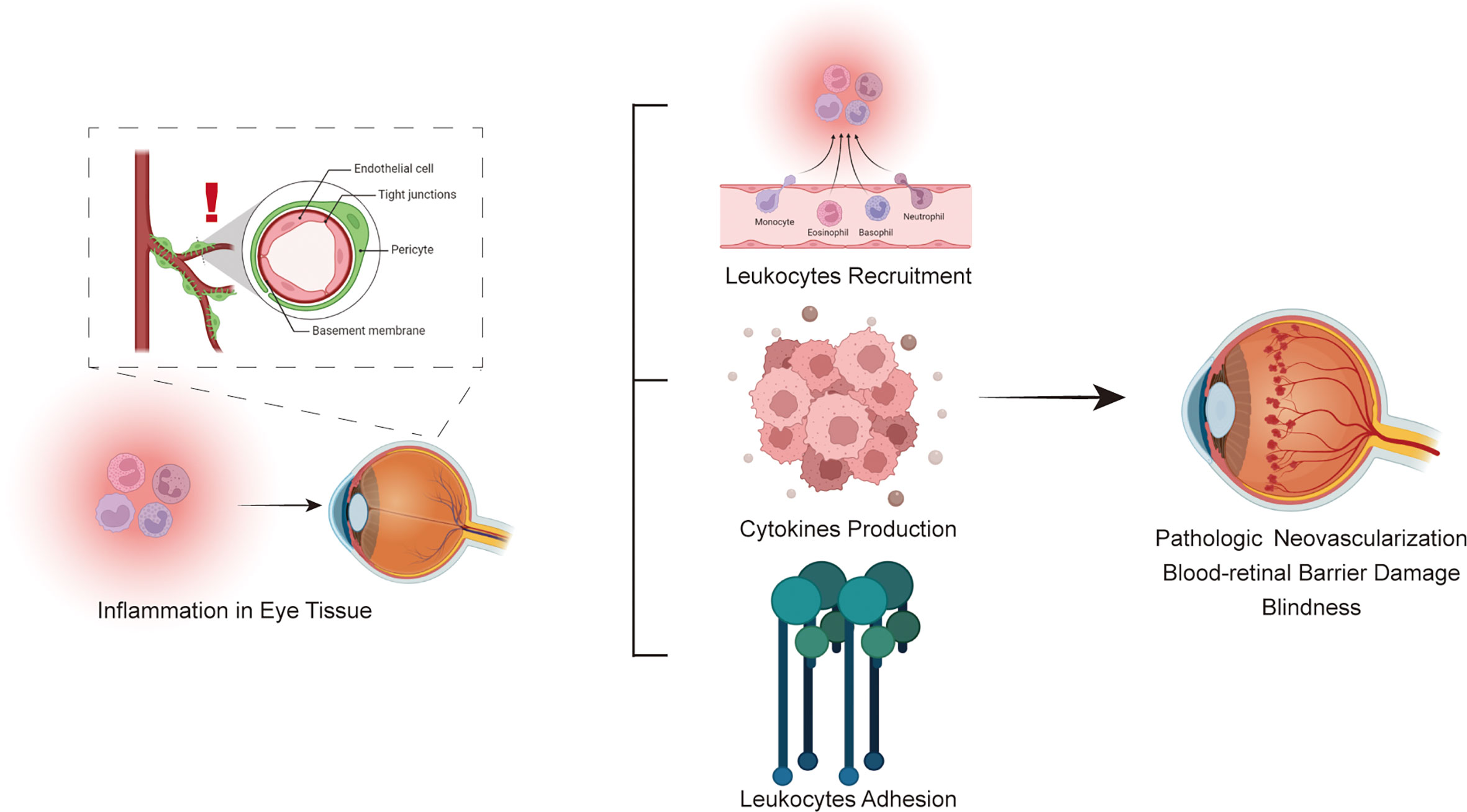
One of the most intriguing aspects of recent studies is the discovery that some patients with PDR show a predominance of immune cells in the vitreous. These cells, including a mix of monocytes, macrophages, T cells, and mast cells, exhibit gene expression patterns that suggest a natural defense mechanism aimed at eliminating aberrant blood vessels. Although the idea of the body defending itself by clearing damaged vasculature seems like a promising therapeutic target, the reality is that this process is often accompanied by a complex series of reactions that trigger additional issues, such as inflammation and fibrosis.
The notion that immune cells can contribute to retinal repair is not entirely new. Previous studies have noted spontaneous regression of neovascularization in some diabetic patients. However, these episodes are typically brief and tend to be overpowered by the continuing metabolic stress of diabetes. The research under discussion compared monocytes from patients with that from non-diabetic donors and found significant differences in gene expression—specifically, enhanced pathways for extracellular trap formation and phagosome formation indicative of vessel clearance.
- Key immune cells involved: Monocytes, macrophages, T cells, mast cells.
- Gene expression highlights: Upregulation of pathways such as neutrophil degranulation and extracellular trap signaling.
- Potential consequence: While the clearance of pathological vessels may reduce neovascularization, this process might also lead to the formation of fibrotic membranes that pose their own set of problems.
This immune-based approach, while promising, is also loaded with issues. The clearance process can be overwhelming in some patients, making it a double-edged sword that requires careful modulation. As we take a closer look at these cells, it becomes clear that tailoring treatment requires us to get into the fine shades of how the immune system both protects and potentially harms the retina.
Endothelial Mitochondrial Dysfunction: A Metabolic Crisis
In other patients, the evidence points to a very different pathology where the endothelial cells themselves suffer from mitochondrial dysfunction. This dysfunction results in a distinct molecular signature that is indicative of compromised energy production and oxidative stress. Unlike the immune-mediated clearance mechanism, these changes suggest that the primary insult comes from within the vascular cells themselves, leading to a breakdown in the maintenance of the blood-retina barrier and further progression of PDR.
Mitochondrial dysfunction in endothelial cells is a particularly nerve-racking finding because it signals that even though anti-VEGF therapies succeed in suppressing vascular leakage, they may not address the underlying metabolic failures. The research used comparative gene expression analyses between healthy retinal endothelial cells and those derived from PDR samples. This comparison revealed significant differences, especially in pathways responsible for oxidative phosphorylation and respiratory electron transport.
To summarize the differences between the immune-driven and mitochondrial dysfunction-driven PDR subtypes, consider the following table:
| Subtype | Dominant Mechanism | Clinical Implication |
|---|---|---|
| Immune-Mediated PDR | Enhanced immune cell activity aimed at clearing pathological vessels | Potential for vessel regression, but risk of inflammation and fibrosis |
| Mitochondrial Dysfunction PDR | Endothelial cell metabolic crisis due to impaired mitochondrial function | Persistent abnormal vessel formation not directly addressed by anti-VEGF therapies |
The Role of Anti-VEGF Therapy and Its Wider Impacts
Anti-VEGF agents have transformed the treatment landscape for diabetic retinopathy by reducing vascular leakage and neovascularization. Yet, as our understanding of the PDR subtypes deepens, it is apparent that these therapies might only address a subset of the underlying problems. Modern research shows that anti-VEGF does more than just curb blood vessel growth—it also affects gene expression in both endothelial and immune cells.
Beyond the Blood Vessels: Off-Target Effects of Anti-VEGF Agents
One of the surprising observations is that anti-VEGF therapy is not confined to reducing endothelial activity. In patients receiving preoperative anti-VEGF injections, there were changes in the transcription programs in immune cells, too. This dual impact suggests that anti-VEGF agents might be influencing the broader retinal environment. In some cases, they suppress inflammation by downregulating inflammatory pathways such as S100 family signaling and STAT3 pathways.
This finding is critical for two reasons. First, it provides new avenues to develop therapies that could harness the beneficial off-target effects while mitigating the potential downsides. Second, it calls attention to the need for considering the whole microenvironment of the retina when designing future treatments.
- Direct effects: Reduction of angiogenesis by targeting VEGF signaling.
- Indirect effects: Modulation of immune responses and inflammatory pathways.
- Clinical challenge: Balancing anti-VEGF benefits with the risk of promoting fibrosis in endothelial cells.
Given these observations, it becomes clear that a one-size-fits-all approach may not be adequate. Both the immune and endothelial components need to be factored into treatment strategies.
Implications for Treatment Strategy
The wider influences of anti-VEGF therapy underscore the need for a multi-pronged approach to diabetic retinopathy management. In patients whose disease is driven primarily by mitochondrial dysfunction, targeting metabolic pathways may prove more effective than simply curbing VEGF activity. Conversely, in patients where immune activity is predominant, modulating the inflammatory response could help manage the progression of PDR.
Here are some potential strategic approaches that emerge from these findings:
- Combination Therapies: Combining anti-VEGF agents with drugs that target mitochondrial health or modulate immune responses could address both sides of the equation.
- Personalized Medicine: By using gene expression profiling, clinicians could determine whether a patient’s PDR is more immune-driven or metabolic in nature and tailor treatments accordingly.
- Nutritional Interventions: Considering the role of oxidative stress, diets rich in antioxidants and mitochondrial support might prove beneficial as adjunctive therapies.
These approaches bring us a step closer to managing the confusing bits of PDR. They are a reminder that understanding the little details behind the disease can help healthcare providers steer through the tangled issues and develop more precise treatment regimens.
Bridging the Gap: From Preclinical Models to Clinical Reality
Animal models, such as the murine oxygen-induced retinopathy (OIR) model, have been invaluable in studying the processes of retinal neovascularization. However, recent data suggest that the OIR model does not fully capture the mitochondrial dysfunction observed in clinical cases of PDR. While the OIR model accurately recapitulates many features of pathological angiogenesis, development-related changes dominate and do not mirror the metabolic struggles of adult diabetic retinas.
Limitations of the OIR Model
The murine OIR model is often used to test new therapeutic targets before moving into human clinical trials. Although it offers useful insights into the angiogenic process, it falls short when it comes to mimicking the long-term metabolic stress experienced by patients with diabetes. The key differences include:
- Developmental Context: The OIR model involves immature retinal vasculature, which is undergoing development, unlike the mature vessels seen in chronically stressed diabetic retinas.
- Metabolic Stress: In diabetic retinopathy, prolonged high blood sugar levels lead to complex mitochondrial insults—a factor that is not replicated in the OIR model.
- Animal-Human Differences: Gene expression changes in animal models do not always align with those seen in human studies, particularly aspects like immune signaling and mitochondrial activity.
The table below highlights some of these differences:
| Parameter | OIR Model | Human PDR |
|---|---|---|
| Vascular Maturity | Immature, developmental vessels | Mature vessels under prolonged metabolic stress |
| Mitochondrial Function | Little evidence of mitochondrial dysfunction | Clear mitochondrial impairment and metabolic dysregulation |
| Immune Environment | Primarily angiogenic signals | Mixed signals: clearance of vessels vs. inflammatory response |
Moving Toward More Realistic Models
In order to develop therapies that truly address the nutty constituents of PDR in humans, future research must look into refining animal models or developing new systems that better replicate the clinical picture. This might include:
- Chronic Disease Models: Long-term animal studies that simulate the chronic hyperglycemia and oxidative stress seen in diabetes could offer more realistic insights.
- Advanced Gene Profiling: Incorporating human gene expression data into animal model research may help identify molecular targets common to both systems.
- Alternative Approaches: Exploring in vitro models using human retinal cells could allow researchers to assess mitochondrial function and immune interactions in a controlled setting.
By developing more robust preclinical models, we can better understand the fine shades of PDR pathology and improve the likelihood that newly developed therapies will be successful in clinical trials.
Exploring Alternative Therapeutic Strategies
Given the layered challenges and nerve-racking realities of PDR, it is clear that our current treatment paradigm – largely dominated by anti-VEGF therapies – may not suffice for all patients. This situation calls for fresh approaches that integrate the key findings from recent studies with complementary strategies, ranging from pharmaceutical interventions to lifestyle modifications.
Combination Therapies for a Multifaceted Disease
One promising avenue involves the use of combination therapies. Instead of relying solely on anti-VEGF agents, clinicians might consider pairing these with medications that address either mitochondrial dysfunction or immune dysregulation. For example:
- Mitochondrial Support Agents: Drugs that enhance mitochondrial function or reduce oxidative stress may help improve the energy balance in endothelial cells. Such agents could include antioxidants, mitochondrial biogenesis stimulators, or metabolic modulators.
- Immunomodulatory Drugs: Targeted therapies that fine-tune the immune response without causing excessive suppression might help steer the process of vessel clearance in a controlled manner. Medications such as selective corticosteroids or emerging biologics that target specific inflammatory mediators may offer benefits.
This multitargeted approach would not only address the obvious angiogenic issues but also work on the super important metabolic and immune components that are integral to the progression of PDR.
The Role of Nutritional and Lifestyle Interventions
No examination of diabetic retinopathy can be complete without considering the potential impact of nutrition and lifestyle changes. Although these interventions might seem off-putting at first, they represent an essential and sometimes overlooked tool in the therapeutic arsenal. Diets enriched in antioxidants, omega-3 fatty acids, and mitochondrial-supporting nutrients (like B vitamins and coenzyme Q10) could help improve vascular health.
Some key recommendations include:
- Antioxidant-Rich Foods: Incorporating colorful fruits and vegetables can provide antioxidants that help neutralize free radicals.
- Omega-3 Fatty Acids: Fish oil and flaxseed oil support vascular health and reduce inflammation.
- Lifestyle Modifications: Regular exercise, weight management, and tight glycemic control are super important measures to mitigate the ongoing metabolic stress that drives mitochondrial dysfunction.
Integrating these nutritional strategies with medical treatments may help dial down some of the nerve-racking challenges presented by PDR.
Emerging Alternatives: Beyond VEGF
Given the limitations of anti-VEGF therapy, there is a growing interest in exploring alternative molecular targets. Research has identified several anti-VEGF-regulated genes that could potentially serve as new therapeutic targets, addressing the small distinctions that remain uncharted. These targets include genes involved in extracellular matrix organization, ion transport, and metabolic regulation.
For example, some of the promising new therapeutic avenues include:
- Angiopoietin-2 Inhibition: With angiopoietin-2 already a target for FDA-approved therapies in other ocular conditions, there is potential to extend this approach to PDR.
- ECM Remodeling Modulators: Since extracellular matrix reorganization is a critical factor in both immune-mediated and mitochondrial dysfunction-driven PDR, molecules that affect ECM components may help alleviate fibrotic complications.
- Ion Channel Regulators: Targeting novel genes that control ion transport and endothelial cell junction integrity could help improve vascular stability and reduce leakage.
These emerging targets provide hope that future therapies might be both more effective and better tailored to the specific subtype of diabetic retinopathy that a patient exhibits.
Integrating Research with Clinical Practice
As we navigate through the tangled issues of diabetic retinopathy, it is critical that clinical practice integrates the evolving scientific knowledge with day-to-day patient management. The advancement in genomic and transcriptomic profiling provides clinicians with key insights into patient-specific disease processes, potentially paving the way for personalized treatment regimens.
Personalized Medicine in Diabetic Retinopathy
Personalized medicine is no longer a futuristic concept—it is rapidly becoming a super important component of modern healthcare. In PDR, the ability to profile the molecular signature of a patient’s retinal cells may soon allow for the customization of therapy. By determining whether a patient’s retinal pathology is predominantly immune-driven or related to mitochondrial dysfunction, clinicians can choose a more directed therapeutic approach.
The steps toward personalized medicine in diabetic retinopathy might include:
- Genetic Profiling: Using RNA sequencing or similar techniques to identify the gene expression patterns in retinal cells.
- Biomarker Identification: Finding key markers—such as indicators of mitochondrial stress or heightened immune activity—that can guide treatment decisions.
- Targeted Therapy: Designing treatment regimens that specifically address the identified molecular derangements, whether they be metabolic, immunologic, or a hybrid of both.
This approach requires a close collaboration between the laboratory and the clinic, ensuring that advances in research directly translate into better outcomes for patients.
Challenges in Translating Research into Practice
Despite the promise of these new approaches, there are several nerve-racking challenges that must be overcome. Among these are the variability in patient responses to therapy, the limited sample sizes in many studies, and the inherent differences between animal models and human disease. All of these factors contribute to the tangled reality that healthcare providers face when trying to implement the latest findings.
Key challenges include:
- Heterogeneity of Disease: Not all patients exhibit the same gene expression profiles, making it difficult to establish universal treatment protocols.
- Model Limitations: As already discussed, the OIR model does not entirely capture the metabolic dysfunction seen in human PDR.
- Clinical Implementation: High costs and the need for specialized equipment to perform in-depth molecular analyses can be overwhelming for many clinics.
While these obstacles are significant, the continued convergence of research and clinical practice holds promise. With further investment in personalized diagnostics and a broader understanding of the fine points of PDR pathology, it should eventually be possible to design therapies that are as individual as the patients themselves.
Future Directions and the Path Ahead
The journey toward improved management of diabetic retinopathy is far from over. As we take a closer look at the results emerging from the latest research, it becomes evident that a combination of immune modulation, mitochondrial support, and targeted anti-VEGF strategies may offer the best path forward. Equally important is the need to incorporate lifestyle and nutritional interventions into the treatment paradigm.
Collaborative Efforts in Research and Clinical Care
The future of diabetic retinopathy treatment lies in collaborative efforts that span the fields of molecular biology, clinical research, and patient care. Researchers must continue to dig into the fine details of the disease, while healthcare providers must remain agile in adapting to new findings. This collaboration will ensure that both the small distinctions in molecular signatures and the broader clinical picture are taken into account when designing treatment strategies.
Some concrete steps that could accelerate progress include:
- Multi-Center Clinical Trials: Larger studies that incorporate diverse patient populations can help validate molecular subtyping of PDR and test targeted therapies.
- Integrative Data Platforms: Creating shared databases that combine genomic, transcriptomic, and clinical data will allow researchers to poke around for new patterns and therapeutic targets.
- Interdisciplinary Conferences: Bringing together experts from different fields—ophthalmology, immunology, molecular biology, and nutrition—can foster innovative ideas and cross-pollination of research findings.
Embracing the Complexity of Diabetic Eye Disease
One of the most invigorating lessons from recent research is the realization that diabetic retinopathy is not a one-dimensional disease. Its complicated pieces—ranging from immune-mediated vessel clearance to mitochondrial dysfunction in endothelial cells—demand a flexible and nuanced approach. There is no simple answer, and the small twists and turns in the data underscore that both types of pathology can coexist even within a single patient.
By recognizing and embracing these multiple facets, the medical community can better design treatment protocols that are both effective and personalized. In turn, this will also help reduce the nerve-racking uncertainty that accompanies current treatment strategies, particularly for those patients who do not respond fully to anti-VEGF therapy.
Conclusions: Charting a New Course in Diabetic Retinopathy Management
Diabetic retinopathy remains one of the most challenging complications of diabetes, riddled with problems that force us to get into the fine points of both the immune and metabolic aspects of the disease. The emerging evidence suggesting two distinct subtypes—one driven by immune defense mechanisms and the other by endothelial mitochondrial dysfunction—offers a refreshing perspective on how we might retool treatment strategies.
Anti-VEGF therapies have been a breakthrough, yet they fall short in addressing the full spectrum of the disease. As we work through the tangled issues using combination therapies, personalized medicine, and nutritional interventions, it becomes increasingly clear that an integrative approach is necessary.
The path ahead involves bridging the gap between preclinical models and clinical realities by refining our models and embracing advanced diagnostic techniques. Although the journey may be intimidating and full of twists and turns, the potential for significantly improved patient outcomes makes it a challenge worth tackling.
In closing, the future of diabetic retinopathy management depends on our ability to weave together cutting-edge research with practical clinical strategies. By staying attuned to both the immune and metabolic dynamics at play, we can develop therapies that not only curb neovascularization but also tackle the root causes of mitochondrial stress and inflammatory damage.
Ultimately, the evolution of diabetic retinopathy treatment will be defined by a collaborative effort among researchers, clinicians, and patients alike. With continued investment in innovative research, personalized diagnostics, and integrative care, we stand on the brink of a new era—one where the confusing bits of PDR can be managed more effectively, restoring hope and vision to those affected by this challenging disease.
Originally Post From https://www.nature.com/articles/s41392-025-02448-9
Read more about this topic at
The Dichotomous Role of Inflammation in the CNS
Mitochondria in innate immune signaling – PMC