Revolutionary Breakthrough in Gene Therapy for AMD: A New Era in Ocular Health
The field of ophthalmology is experiencing a remarkable transformation thanks to emerging gene therapies that promise a long-term solution for age-related macular degeneration (AMD). As a condition that affects millions worldwide, AMD has long been one of the most nerve-racking challenges for both patients and healthcare professionals. New developments using adeno-associated virus (AAV) vectors to deliver bispecific antibodies may soon change that narrative. In this opinion editorial, we take a closer look at this breakthrough, examining the promise of gene therapy, the tricky parts involved in its development, and the implications for patient care in the years ahead.
A Fresh Perspective on Age-Related Macular Degeneration
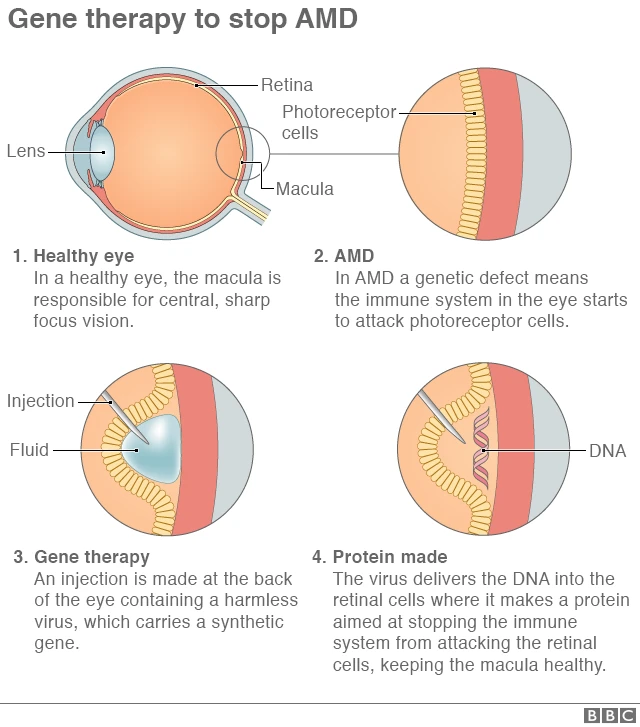
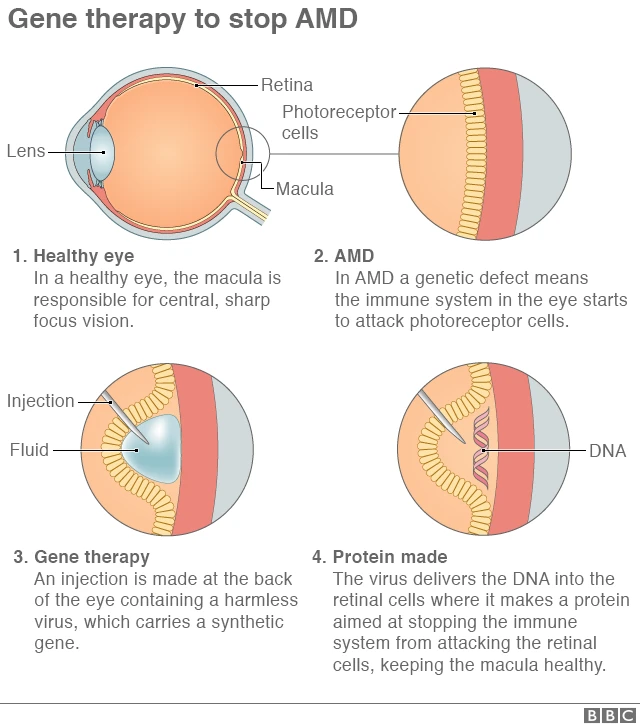
Age-related macular degeneration is a leading cause of irreversible vision loss, particularly among older adults. The condition is marked by abnormal blood vessel growth and vascular leakage in the retina. Over time, these changes can lead to blurred or lost vision—a reality that is both overwhelming and intimidating for those affected.
Traditional treatments typically involve repeated intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents. While effective to a degree, these treatments are not without their tangled issues, most notably the need for frequent clinic visits and invasive procedures. Many patients find this process off-putting, given its nerve-racking nature and the significant time commitment required.
Gene Therapy Emerges as a One-Time, Long-Term Solution
Recent developments in gene therapy for AMD offer hope for a one-and-done treatment approach. Scientists have engineered an AAV vector—known as AAV-RPE—that is capable of delivering a bispecific antibody to retinal pigment epithelium (RPE) cells. This antibody simultaneously targets vascular endothelial growth factor-A (VEGF-A) and angiopoietin-2 (ANG-2), aiming to prevent the abnormal blood vessel growth that leads to vision loss.
Unlike traditional therapy methods, the gene therapy product, identified under the designation XMVA09, has been designed to provide long-lasting expression of the therapeutic antibody. Early trials in animal models such as mice and non-human primates have shown encouraging results, and human clinical studies have reported that the therapy is well-tolerated. The potential to reduce or eliminate the need for frequent invasive injections marks a significant step forward in managing this complicated piece of a condition that has historically been full of problems.
The Science Behind the Treatment: A Dive into the Little Details
Let’s dig into the nitty-gritty of this pioneering treatment. At its core, the therapy harnesses the power of an engineered AAV vector. Here’s a simplified breakdown of the process:
- Vector Engineering: The AAV is modified to express a bispecific antibody. This dual-targeting molecule binds to both VEGF-A and ANG-2, effectively reducing the stimuli that cause abnormal blood vessel growth.
- Long-Term Expression: Once introduced into the eye via an injection, the vector integrates into retinal cells, providing continuous production of the therapeutic antibody over a prolonged period.
- Reduced Treatment Frequency: This continuous expression aims to mitigate the need for the nerve-wracking frequent treatments that many patients currently endure.
The table below summarizes the key components and advantages of this gene therapy approach:
| Component | Description |
|---|---|
| AAV Vector | An engineered virus used as a delivery agent for the therapeutic gene |
| Bispecific Antibody | A molecule that binds both VEGF-A and ANG-2 to inhibit pathological processes in AMD |
| Long-term Expression | Continuous production of the antibody from retinal cells post-injection |
| Treatment Burden | Reduction in the need for frequent intravitreal injections |
By providing a deeper look into the hidden complexities of this technology, one can appreciate the innovative thinking behind using gene therapy as a long-term solution for AMD.
Addressing the Tricky Parts: Safety and Efficacy Considerations
One of the primary concerns that healthcare professionals and patients alike have is safety. Gene therapies in general have a reputation for being intimidating due to their complexity and the associated regulatory challenges. However, the encouraging preliminary data suggests that XMVA09 is well-tolerated in clinical trials. Yet, as with any revolutionary treatment, several tricky parts must be considered:
- Long-Term Safety: While early studies are promising, extended observation is necessary to ensure that the continuous expression of the therapeutic antibody does not lead to unforeseen complications.
- Immunogenicity: There is a potential for the body’s immune system to react against the AAV vector or the expressed protein. Researchers are carefully assessing these risks to ensure that the therapy remains effective over time without triggering adverse immune responses.
- Delivery Challenges: Even though the therapy is intended as a one-time injection, ensuring that the vector effectively reaches and integrates into the right cells is a delicate process. There are a number of fine points in making sure that dosing and delivery are optimized.
Healthcare experts emphasize that these issues, while tricky, are not insurmountable. Ongoing clinical trials are designed to address these concerns head-on. It is critical to continuously monitor the delicate parts of the therapy’s performance, all while navigating the tangled issues of ensuring long-term safety and efficacy.
Alternative Approaches vs. Gene Therapy: A Comparative Analysis
When comparing current treatments for AMD with the promise of gene therapy, several differences stand out. Traditional anti-VEGF treatments have undoubtedly improved patient outcomes, but they come with their own set of challenges:
- Frequent Injections: The need for regular clinic visits and invasive procedures can be both time-consuming and off-putting.
- Variability in Response: Not every patient responds equally well to conventional therapies, with outcomes often varying due to subtle differences in individual biology.
- Cost and Accessibility: Repeated treatments can result in high long-term costs for patients and healthcare systems alike.
In contrast, gene therapy holds the promise of a one-time injection that could potentially provide decades of benefit. While we should not overlook the potential pitfalls and the nerve-wracking uncertainties associated with new therapies, the shift toward targeted gene therapy could revolutionize the way we manage AMD.
Here are some key comparisons in a tabular format:
| Aspect | Traditional Anti-VEGF Therapy | Gene Therapy Approach |
|---|---|---|
| Treatment Frequency | Multiple injections required regularly | One-time injection with long-term expression |
| Treatment Invasiveness | Repeated invasive procedures | Single invasive procedure |
| Patient Compliance | Often low due to frequent visits and discomfort | Potentially higher compliance with sustained effects |
| Cost Over Time | High cumulative cost | Potentially lower long-term costs |
Economic Implications: Balancing Innovation with Affordability
The financial dynamics surrounding new gene therapies are a critical, though sometimes confusing, piece of the overall picture. While the promise of a one-time treatment appeals greatly both to patients and healthcare systems, the development and manufacturing of such therapies are loaded with challenges that can drive up costs.
Some key economic considerations include:
- Research and Development Costs: The intricate process of developing gene therapies comes with a hefty price tag. Investment in R&D is super important to push these treatments from the lab to the clinic.
- Manufacturing Complexities: Producing clinical-grade viral vectors and ensuring their safety on a mass scale is full of tricky parts that require cutting-edge technology and infrastructure.
- Pricing Models: Pharmaceutical companies must balance the recouping of their investments with the need to make these therapies accessible. This balancing act can sometimes result in high initial prices.
- Reimbursement Policies: Healthcare payers and insurance companies will need to adjust to the new paradigm, rethinking reimbursement models when faced with a one-time, potentially curative intervention.
Though some of these issues may appear overwhelming at first glance, it is essential that stakeholders work together to figure a path that ensures both innovation and affordability. After all, the ultimate goal is to extend the benefits of this groundbreaking therapy to as many patients as possible.
Regulatory and Logistical Bumps on the Road Ahead
Gene therapy in itself is a field that is full of problems when it comes to regulatory approval. Despite the excitement surrounding new treatment modalities like XMVA09, there are a number of regulatory hurdles that must be cleared before such therapies become widely available.
Key factors include:
- Stringent Clinical Trials: Regulatory bodies require comprehensive data demonstrating both safety and efficacy. This process can be intimidating for companies, with fine shades in the data driving improvements in trial design.
- Long-term Follow-up: Ongoing monitoring of patients over extended periods is necessary to ascertain that there are no delayed adverse effects—a process that is both nerve-wracking and resource-intensive.
- Manufacturing and Quality Control: Ensuring batch consistency and clinical-grade purity continues to be a challenging bit in the development cycle, necessitating investments in specialized facilities and expert personnel.
- International Coordination: Export restrictions—such as those recently affecting semiconductor availability—can disrupt not only production timelines but also increase the cost of manufacturing and distribution.
Despite these twists and turns, the potential benefits for patients cannot be overstated. Regulatory agencies worldwide are becoming better equipped to evaluate these advanced therapies, acknowledging that the rewards may far outweigh the risks when it comes to treating a disease as convoluted and debilitating as AMD.
Patient Perspectives: How Would This Change the Daily Burden?
For patients living with AMD, the daily reality of coping with vision loss involves many daunting moments. The fear of deteriorating vision, combined with the stress of managing frequent treatments, creates a scenario that is understandably off-putting. The promise of a one-shot gene therapy offers a beacon of hope—one that could potentially remove much of the nerve-wracking treatment burden.
Some key benefits from the patient’s perspective include:
- Quality of Life Improvement: Imagine no longer having to schedule and endure repeated injections, thereby freeing up time and reducing anxiety about the future.
- Long-Term Vision Stability: The therapy aims to provide lasting protection against the progression of AMD, which could significantly enhance day-to-day independence.
- Reduced Clinic Visits: A single treatment session means fewer trips to the doctor, leading to a more relaxed and manageable treatment regimen.
- Peace of Mind: Steady, continuous expression of the therapeutic gene gives patients a tangible sense of security that their vision is being safeguarded around the clock.
Patients who have experienced the nerve-racking routine of anti-VEGF injections can especially appreciate the small distinctions between conventional treatments and this new gene therapy. Many report that even the possibility of a prolonged treatment interval can feel liberating, as it removes much of the overwhelming daily anxiety.
The Role of Healthcare Providers in Steering Through Change
Healthcare professionals, too, must make their way through a landscape that is continually evolving. The introduction of gene therapies is compelling but also full of tangled issues that require careful thought and strategic planning. Physicians and ophthalmologists will need to:
- Educate Patients: Explain the benefits and potential risks associated with gene therapy in layman’s terms, ensuring that patients understand the fine details of the treatment process.
- Monitor and Follow-Up: Develop robust follow-up protocols to track the performance of the therapy over time, ensuring that any signs of adverse effects are promptly addressed.
- Collaborate with Researchers: Stay informed about the latest studies and clinical outcomes to help figure a path through the evolving treatment landscape.
- Adapt Practice Models: Evolve traditional treatment paradigms to incorporate new gene-based therapies that could significantly reduce the frequency of patient visits.
This collaborative approach is essential to translate these cutting-edge treatments from research laboratories into everyday clinical practice. By taking a closer look at each fine point, providers can better navigate the tangled issues of implementation while ensuring that patient safety remains super important.
Balancing Expectation with Caution: The Road Ahead
While the promise of gene therapy is undeniably exciting, it is important to remain realistic about the challenges ahead. The road to widespread adoption of this treatment is filled with both potential and pitfalls. It is critical to manage expectations by acknowledging that:
- There is still much to learn about long-term safety and effectiveness.
- Regulatory and logistical challenges may slow the pace of adoption.
- Economic factors, including cost and reimbursement policies, will play a major role in accessibility.
- Patient education and physician training are essential to ensure a smooth transition from conventional treatments.
These points serve as a reminder that while the gene therapy for AMD shows tremendous promise, the journey from promising studies to everyday clinical practice is loaded with twists and turns. Healthcare professionals, regulators, and industry stakeholders must continue to work together to tackle both the complicated pieces and the challenging bits of this innovative treatment approach.
What Does the Future Hold for Gene Therapy in Ophthalmology?
Looking forward, the potential of gene therapy to change the face of ophthalmology extends far beyond AMD. Researchers and clinicians are already exploring similar strategies for other ocular diseases, including inherited retinal disorders and even diabetic retinopathy. If the early success of treatments like XMVA09 can be replicated and expanded upon, we could be witnessing a paradigm shift in how we treat not only AMD but a host of other vision-impairing conditions.
In an increasingly interconnected world, innovative treatments often spark further discoveries, and the gene therapy approach for AMD is no different. This could set off a cascade of research and development initiatives aimed at refining vector technology, improving manufacturing processes, and ultimately developing even more personalized and effective treatments.
The key factors that will drive the industry forward include:
- Scientific Innovation: Continued research is essential to improve our understanding of the delicate parts of ocular diseases and refine the therapeutic strategies employed.
- Collaboration Across Disciplines: Clinicians, researchers, and industry specialists must work hand in hand to figure a path that addresses both the technical and logistical challenges inherent in these therapies.
- Patient-Centered Development: Engaging with patients to understand their needs and concerns will help tailor treatment approaches that not only extend vision but also greatly enhance quality of life.
- Regulatory Adaptation: Agencies need to balance rigorous safety standards with flexibility to support innovation, ensuring that life-changing treatments can reach patients without undue delay.
Each of these factors is super important not only in the context of AMD but also in paving the way for a future where personalized medicine becomes the standard in ophthalmic care.
Overcoming the Troublesome Aspects: Tackling Manufacturing and Supply Chain Hurdles
Another layer of complexity in bringing gene therapy from the lab to the clinic pertains to the manufacturing and supply chain challenges. From scaling up production to coping with international supply restrictions, the process is full of confusing bits that require careful mitigation strategies. Some of the key hurdles include:
- Production Scalability: Producing high-quality AAV vectors on a large scale is a tricky part that demands advanced biotechnology and extensive quality control measures.
- Supply Chain Dependencies: Recent global issues, such as export limitations on specialized AI chips and semiconductors, have shown that even seemingly peripheral industries can have a ripple effect on the availability of breakthrough medical treatments.
- Cost Implications: The expense associated with scaling up manufacturing processes, while maintaining stringent safety standards, represents one of the more intimidating challenges that companies must face.
To address these challenges, stakeholders are exploring strategies such as establishing regional manufacturing hubs, partnering with technology innovators, and streamlining regulatory approvals. Taken together, these efforts are designed to ensure that the revolutionary benefits of gene therapy remain accessible to patients worldwide.
Alternative Medicine and Nutritional Perspectives: Complementary Approaches in Ocular Care
While gene therapy represents a significant scientific leap, many patients and practitioners continue to advocate for complementary approaches that integrate modern medicine with alternative and nutritional strategies. These additional layers of care are important for managing overall eye health. Some of these strategies include:
- Dietary Modifications: Antioxidant-rich diets and specific nutrients such as lutein and zeaxanthin have been shown to support retinal health. While not a cure, they can help minimize the progression of vision loss.
- Herbal Supplements: Some traditional remedies focus on supporting blood circulation and reducing inflammation, both of which can complement conventional treatments and gene therapies.
- Holistic Patient Care: Integrating nutritional counseling and lifestyle adjustments with cutting-edge gene therapy enables a more rounded treatment approach that addresses both the direct causes of AMD and its broader health impacts.
It is important to note that while these complementary therapies can provide supportive benefits, they are designed to work in tandem with advanced treatments like gene therapy rather than replace them. The fusion of modern biotechnological solutions with time-tested holistic practices reflects an evolving healthcare landscape that is increasingly patient-centered and adaptive.
Charting a New Course: The Impact on Healthcare Systems and Future Trends
The introduction of gene therapy for AMD is poised to have a lasting impact on healthcare systems around the globe. Beyond the obvious patient benefits, these advancements raise important questions about how healthcare infrastructures will adapt. Some notable trends include:
- Shifting Resource Allocation: A move from recurring treatments to one-time therapies may free up clinical resources, allowing providers to concentrate on early diagnosis and other preventative measures.
- Improved Patient Management: A reduction in the frequency of invasive procedures not only improves patient quality of life but also streamlines the scheduling and logistics involved in ophthalmic care.
- Innovation in Treatment Protocols: The success of gene therapy for AMD could serve as a catalyst for new protocols in managing other chronic conditions, inspiring healthcare providers to adopt more sustainable, long-term treatment solutions.
- Global Health Impact: With millions suffering from vision loss due to AMD, successful gene therapy could significantly reduce the global burden of blindness, leading to better overall public health outcomes.
The healthcare landscape is no stranger to complex, tangled issues, but the persistent drive for innovation ensures that these challenges are met with creative and collaborative solutions. As gene therapy continues to mature, we expect to find our way through these obstacles, ultimately fostering an environment where advanced therapeutics become a mainstay in patient care.
Conclusion: A Vision for the Future of Eye Care
In conclusion, the development of gene therapy for age-related macular degeneration is a landmark achievement that offers hope to millions who have long struggled with the daunting daily challenges of vision loss. With continuous research and clinical advancements, what was once a nerve-wracking, never-ending series of hospital visits might soon be supplanted by a one-time, long-lasting treatment that restores, protects, and preserves vision.
Though the journey is filled with twists and turns—ranging from regulatory hurdles to manufacturing challenges—the collaborative efforts of scientists, healthcare providers, and policy makers are shaping a future where innovative treatments are not just a promise but a reality. By taking a closer look at the subtle parts of this transformative process, we gain insights into how gene therapy stands to redefine ocular care.
The potential for this therapy to radically change patient outcomes cannot be understated. From providing a lasting solution with a single injection to easing the economic and logistical burdens on the healthcare system, the benefits of this approach extend far beyond the individual. It is a testament to human ingenuity and the relentless pursuit of solutions that ultimately improve lives.
As we look ahead, it is clear that while challenges remain—be they in the form of manufacturing bottlenecks, regulatory complexities, or economic considerations—the promise of gene therapy for AMD shines brightly. Just as alternative approaches and nutritional strategies offer complementary support to eye health, cutting-edge scientific breakthroughs like this gene therapy serve as a beacon of hope for those caught in the tangled issues of chronic vision loss.
Our journey through the world of gene therapy reveals that modern medicine is not static but ever evolving. Every intricate detail, every fine shade of discovery, and every small distinction between success and setback brings us closer to a future where vision loss is no longer an intimidating prospect, but a manageable and even preventable condition.
In a world where the challenging bits of traditional treatments have often left patients feeling overwhelmed, the one-time gene therapy approach is not just a medical intervention—it’s a paradigm shift. It represents a commitment to finding your path through the maze of modern healthcare challenges and ultimately making life significantly better for millions of people.
As we continue to celebrate such groundbreaking advances, it is crucial that we remain mindful of the work that still lies ahead. Through continued research, collaboration, and patient-centered care, we can steer through the confusing bits and tangled issues of this emerging field. The vision of a future free from the burden of recurrent, invasive treatments is now within our grasp, paving the way for a new era in ocular health—a future that promises clarity, hope, and a renewed quality of life.
Originally Post From https://www.ainvest.com/news/gene-therapy-age-related-macular-degeneration-offers-long-term-solution-2508/
Read more about this topic at
Infant with rare, incurable disease is first to successfully …
World’s First Patient Treated with Personalized CRISPR …