Engineered Exosomes: A Game-Changer for Ocular Vascular Diseases?
The world of ocular medicine is facing a revolution. In recent years, tiny vesicles called exosomes have emerged from the lab bench into the spotlight as promising vehicles for drug delivery. These natural, cell-derived carriers are now being engineered to combat ocular vascular diseases such as age-related macular degeneration, diabetic retinopathy, and other conditions that cause neovascularization in the eye. In this opinion editorial, we take a closer look at the promise, the tricky parts, and the subtle details of these innovative therapies while exploring the potential future of engineered exosomes in ophthalmology.
Tangled Issues in Ocular Neovascular Conditions
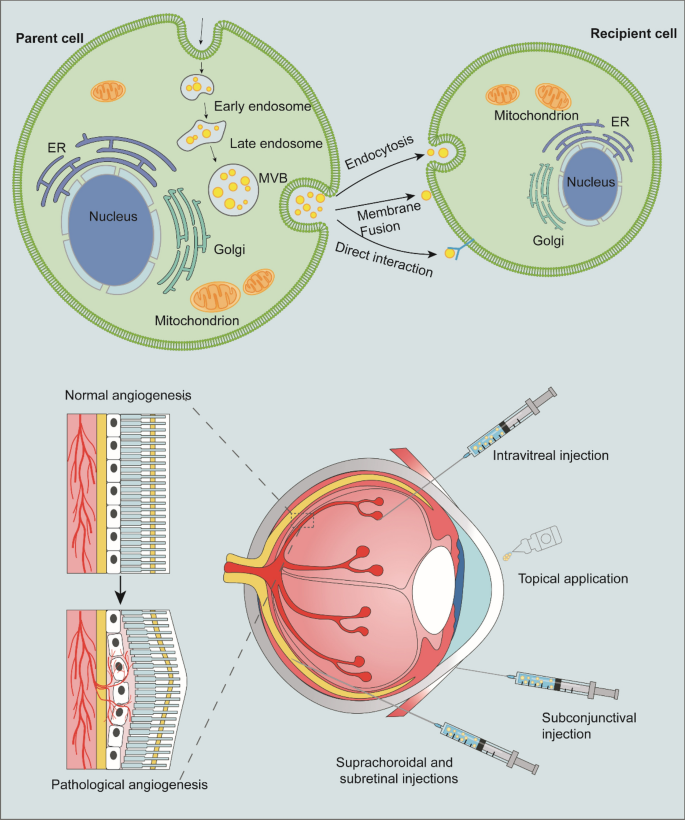
Ocular neovascular diseases are a major cause of irreversible vision loss around the world. Under hypoxic conditions and chronic inflammation, the eye’s delicate blood vessels may undergo uncontrolled growth, leading to fluid leakage, scarring, and vision impairment. Traditional treatments have relied on anti-vascular endothelial growth factor (anti-VEGF) drugs, but these therapies are loaded with problems. They require frequent injections that many patients find nerve-racking, and they do not fully address the underlying tissue repair processes.
Recent research points to engineered exosomes as potential alternatives. Unlike synthetic nanoparticles, exosomes are natural messengers capable of delivering proteins, RNA, and other biomolecules directly to target cells with super important precision. Their ability to traverse the blood–retinal barrier, coupled with low immunogenicity, makes them ideal candidates in our fight against medical conditions that hinge on abnormal angiogenesis and chronic inflammation.
Fine Points in Exosome Biology and Therapeutic Potential
Engineered exosomes offer a unique blend of natural compatibility and customizable design options, making them a promising tool for treating retinal diseases. On one hand, these extracellular vesicles carry essential cargo from their parent cells, which can trigger specific responses in the recipient cells. On the other, their inherent characteristics—such as small size, biocompatibility, and the ability to cross biological barriers—make them attractive carriers as compared to conventional drug delivery methods.
For example, by loading exosomes with anti-VEGF molecules or anti-inflammatory microRNAs, scientists can potentially shut down the pathways that lead to aggressive blood vessel formation. In addition, engineered exosomes can be modified on their surface with targeting peptides (like RGD motifs) to ensure they find their way to the pathological tissue, reducing off-target effects and enhancing drug efficacy.
Overcoming Intimidating Ocular Delivery Barriers
One of the nerve-racking challenges in ocular medicine is finding your way through the eye’s protective barriers. The blood–retinal barrier (BRB) and the corneal epithelium are designed to protect delicate tissues from outside interference. However, these same barriers can also block therapeutic agents, severely limiting treatment effectiveness.
Engineered exosomes, thanks to their minute size and natural cell-derived composition, are proving capable of finding a path across these obstacles. Researchers have reported that exosomes can effectively infiltrate retinal layers via intravitreal injection or even through topical applications when coupled with cell-penetrating peptides. This development is a real boon for patients who might otherwise face overwhelming injection frequencies.
Digging into the Nitty-Gritty of Exosome Engineering
Let’s take a closer look at the fine details of engineering exosomes for ocular therapy. One of the most interesting aspects is how these vesicles are loaded with therapeutic cargo. Methods like electroporation, sonication, and chemical conjugation are used to incorporate drugs, peptides, or RNA molecules into exosomes, each coming with its own set of twisting challenges.
For instance, electroporation uses electrical pulses to create temporary pores in the exosome membrane that allow cargo to seep in. While this method boasts high loading efficiency, it can also disrupt the integrity of the vesicle if not carefully managed. On the other hand, sonication, which uses sound waves, can be gentler but sometimes results in vesicle aggregation. The choice of method often depends on balancing loading efficiency with the need to maintain exosome stability.
Bullet-Point Summary: Pros and Cons of Current Exosome-Loading Techniques
- Electroporation: High loading yields, but can damage membranes if overdone.
- Sonication: Gentle on the vesicle structure, with moderate loading efficiency; potential for aggregation.
- Chemical Conjugation: Provides stable bonding of therapeutic molecules, yet may require complex purification steps.
The table below summarizes these techniques for clarity:
| Method | Advantages | Challenges |
|---|---|---|
| Electroporation | High cargo loading; Effective for RNA delivery |
Potential membrane disruption; Requires optimization |
| Sonication | Maintains membrane integrity relatively well; Simple procedure |
Risk of vesicle aggregation; Moderate loading ease |
| Chemical Conjugation | Stable attachment of cargo; Long-term release potential |
Complex purification steps; Possibly reduced bioactivity |
Managing Your Way Through Manufacturing Challenges
Beyond the lab, another layer of difficulty is finding your path through the logistical and regulatory hurdles. Large-scale production of exosomes remains tricky. Unlike conventional drugs, exosomes are heterogeneous by nature; their composition can vary based on the cell source and isolation method. This makes achieving consistency across batches—a critical factor in drug therapy—an ongoing challenge.
Moreover, storing exosomes without degradation or aggregation is full of problems. Temperature variations, pH shifts, and oxidative conditions all threaten the stability of these delicate vesicles. Novel solutions are being developed, including lyophilization and specialized storage buffers, to extend exosome shelf life. However, the entire process—from production and isolation to storage and quality control—requires a level of precision that is both technically and financially demanding.
Sorting Out Regulatory Roadblocks and Quality Control
Along with the engineering twists and turns come regulatory challenges that are on edge with uncertainties. Currently, regulatory agencies are still figuring out how best to classify and oversee exosome-based therapies. Because exosomes straddle the worlds of biologics and drug delivery vehicles, guidelines are both confusing and evolving.
Quality control is another key issue. Consistent production requires not only robust isolation methods but also detailed characterization of exosome size, cargo, and surface markers. In this context, innovative techniques like nanoparticle tracking analysis and electron microscopy have become essential for ensuring that each batch meets the set standards. As these regulatory pathways become clearer, the hope is that safe, effective, and scalable exosome therapies will soon make the leap from research to everyday clinical use.
Innovative Combination Therapies and Personalized Approaches
Engineered exosomes are not viewed as a stand-alone magic bullet; instead, they can complement existing therapies. Combining the targeted delivery capabilities of these vesicles with traditional anti-VEGF drugs could reduce injection frequencies and improve patient comfort.
Moreover, there is growing interest in personalized medicine that leverages the patient’s own cellular material. Autologous exosomes—those isolated from a patient’s own cells—could be re-engineered to carry custom therapies tailored to individual genetic or molecular profiles. Such personalized approaches could help address the little twists unique to each patient’s disease, ensuring that treatment is as specific as it is effective.
Small Distinctions: Targeting Specific Ocular Tissues
A key advantage of exosome-based drug delivery is their capability to bridge subtle differences between various ocular tissues. It turns out that different parts of the eye may require different therapeutic approaches. For example, the retinal pigment epithelium (RPE) is crucial for maintaining the integrity of the retina, while vascular endothelial cells in the choroid are central to neovascular processes.
By engineering exosomes with surface ligands that specifically bind to receptors on these distinct cell types, researchers can fine-tune the delivery of medication. This kind of targeted therapy not only improves drug bioavailability but also minimizes unintended interactions with cells outside the affected region, thereby reducing side effects. Such detail-oriented strategies are super important to the future of precise ophthalmic treatment.
Exploring the Use of Long-Acting Exosome Platforms
Sustained-release methodologies are another area where engineered exosomes show real promise. Imagine a self-regulated delivery system that slowly releases therapeutic agents over many weeks. This would be a huge leap forward compared to the current nerve-racking schedule of monthly or even bi-weekly injections that many patients endure.
Recent advances in biomaterial integration have led to the development of hybrid systems, where exosomes are combined with degradable polymers. These innovative smart platforms offer the dual benefits of precise targeting and extended release. Not only do they boost the duration of the therapeutic effect, but they also provide a “set-and-forget” framework that significantly improves patient compliance and overall quality of life.
Emerging Applications Beyond Ocular Neovascular Disorders
While engineered exosomes are frequently discussed in the context of retinal diseases, their therapeutic scope is much broader. Researchers are also investigating their potential in treating other ocular challenges such as corneal injuries, glaucoma, and even retinal degenerative disorders. The adaptable nature of exosomes allows them to be customized for a wide range of clinical applications, making them a truly versatile tool in modern medicine.
For instance, in corneal graft rejection—a condition marked by a tense immune response—exosomes derived from immunosuppressive mesenchymal stem cells have shown promise in modulating local immunity. Similarly, in glaucoma, exosome therapies might be used to protect retinal ganglion cells while improving the drainage of intraocular fluids. These varied applications underscore the super important potential of engineered exosomes as a multifaceted therapeutic platform.
Lessons from the Research: A Closer Look at Recent Findings
Recent studies have consistently shown that the tiny vesicles can reduce pathological angiogenesis. Preclinical research indicates that exosomes wrapped with anti-angiogenic proteins or microRNAs can significantly reduce new blood vessel proliferation in animal models. Clinical research is uncovering subtleties in dosage and administration frequency while highlighting the importance of a controlled manufacturing process.
For example, one set of studies focused on the use of exosome-loaded anti-inflammatory molecules to lower the expression of key cytokines. These therapies not only inhibited the growth of new vessels but also helped to restore the normal function of the blood–retinal barrier. Such dual-action approaches are proving especially promising because they address both the visible symptoms and the underlying causes of ocular diseases.
Neutral Perspectives on Risk: Balancing Promise with Caution
Though the excitement surrounding engineered exosomes is notable, it is important to maintain a balanced outlook. The field is not without its scary challenges. Apart from the technical twists involved in manufacturing and isolation, there are lingering concerns about long-term safety, the possibility of off-target effects, and immune system activation in some cases.
Moreover, while early-phase clinical trials have shown a favorable safety profile, more extensive research is needed to confirm these findings in larger patient populations. Researchers and clinicians alike must work together to continuously refine the protocols for exosome engineering, isolation, and storage. Regulatory bodies worldwide are actively engaged in producing guidelines that ensure both efficacy and safety; however, the path remains loaded with issues that must be carefully sorted out before these therapies become mainstream.
Key Steps for the Future Adoption of Engineered Exosome Therapies
Moving forward, several key steps are critical to the broader application of exosome-based therapies:
- Standardization of Manufacturing: Developing reproducible production methods that yield homogeneous exosome populations is essential for clinical success.
- Enhanced Stability and Storage Solutions: Innovations in formulation and storage conditions will help preserve the healthy nature of exosomes during transport and administration.
- Optimized Loading Technologies: Fine tuning methods like electroporation and sonication to maximize cargo incorporation without sacrificing vesicle integrity.
- Rigorous Clinical Trials: Large-scale studies are necessary to clearly establish the safety, optimal dosing, and long-term effects of engineered exosomes.
- Regulatory Frameworks: Clear guidelines must be established by regulatory authorities to manage this emerging technology, ensuring that patients receive treatments that are both safe and effective.
Integrating Engineered Exosomes into a Broader Treatment Paradigm
The advent of engineered exosomes is not a call to discard current treatments entirely; rather, it is an invitation to integrate these novel systems into a multifaceted therapeutic paradigm. In many cases, the best approach may be a combination therapy that leverages the strengths of both exosome-based delivery and established anti-VEGF drugs or corticosteroids.
Such combination strategies are loaded with potential. By delivering drugs in a sustained-release format while simultaneously targeting the specific immune and angiogenic pathways involved, clinicians can offer a more holistic solution for patients with vascular eye diseases. This approach could reduce the number of invasive procedures required, thus easing the burden on patients while enhancing long-term visual outcomes.
Taking the Wheel: Future Perspectives and the Road Ahead
Looking ahead, the future of engineered exosome-based therapies in ocular medicine appears intensely promising. With rapid advances in biotechnology and nanomedicine, many of the current challenging bits—whether they be manufacturing setbacks or regulatory uncertainties—are beginning to be surmounted.
Continued research and collaboration among scientists, clinicians, and regulatory agencies will be key to refining these technologies. The development of exosome hybrids with synthetic nanoparticles, for instance, could further improve drug loading and delivery efficiency. Personalized exosome therapies, tailored to the specific needs of individual patients, might one day become a must-have tool in managing not only ocular diseases but also other complex disorders where precision delivery is critical.
Table: Future Milestones in Engineered Exosome Therapies
| Milestone | Key Areas of Focus | Expected Impact |
|---|---|---|
| Standardized Isolation Techniques | Consistent purification and quality control | Improved reproducibility and safety |
| Enhanced Cargo Loading | Optimization of electroporation, sonication, and conjugation methods | Higher therapeutic payload with preserved vesicle integrity |
| Improved Storage Technologies | Lyophilization and advanced stabilizing agents | Extended shelf life and reduced degradation |
| Regulatory Clarity | Development of unified guidelines | Smoother clinical transition and increased investment |
| Personalized Exosome Design | Customization of surface ligands and cargo based on patient profiling | More precise, patient-specific therapy with better outcomes |
Conclusion: A Neutral Outlook on a Promising Technology
Engineered exosomes have captured the imagination of researchers around the globe and are quickly establishing themselves as one of the most super important tools in the fight against ocular vascular diseases. The ability to load these tiny vesicles with a cocktail of therapeutic agents, combined with the potential to selectively target specific tissues in the eye, offers a promising alternative to conventional treatments that are often intimidating in their frequency and complexity.
While there are still many confusing bits to work through, including manufacturing hurdles, off-target risks, and regulatory uncertainties, the progress made so far indicates that these challenges are not insurmountable. The future will likely see engineered exosomes integrated into combination therapies, personalized treatment strategies, and sustained-release drug delivery platforms that together pave the way for improved outcomes and better quality of life for patients.
In our view, the maze of ocular vascular disease treatment is just beginning to be unraveled as innovative exosome technologies continue to make headway. By maintaining a neutral stance that carefully weighs both the promise and the problematic twists, we can appreciate the transformative potential of this approach while staying realistic about the work that remains. As clinical trials expand and production scales up, it will be exciting to witness how these advances eventually re-shape the landscape of ophthalmic care.
Ultimately, the journey of engineered exosomes—from conceptual breakthroughs to clinical reality—stands as a testament to the ingenuity and persistence of modern biomedical research. With continued investment in translating these technologies, the day may soon come when patients face fewer invasive procedures and enjoy better, long-lasting vision restoration, marking an important advance in the management of ocular vascular disease.
Originally Post From https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-025-03589-3
Read more about this topic at
Engineered exosomes from different sources for cancer …
Engineering Exosomes for Therapeutic Applications


