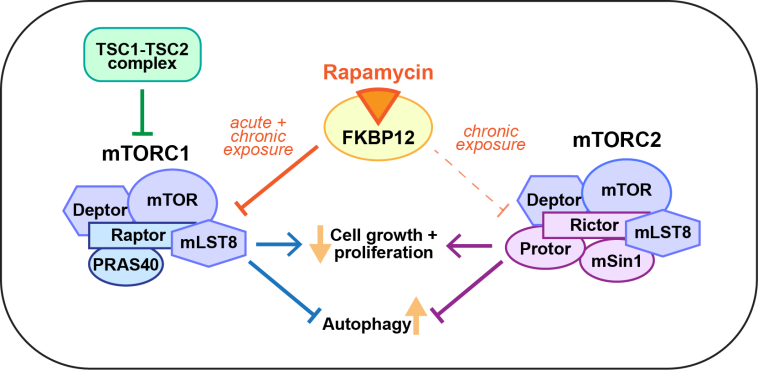
Advancing Treatment for Rare Skin Conditions: The Case of QTORIN Rapamycin
In today’s rapidly evolving landscape of modern medicine, breakthroughs in treatments for rare diseases are not only encouraging but also essential. Palvella Therapeutics, a clinical-stage biopharmaceutical company, has recently announced an expansion of their QTORIN™ 3.9% rapamycin anhydrous gel development program to include a particularly challenging condition—clinically significant angiokeratomas. This news has stirred interest among both clinicians and patients, as it represents a promising step toward addressing a condition where no FDA-approved therapies currently exist.
As an editor committed to exploring both the science and the human impact of rare disease research, it is worthwhile to take a closer look at this development. In this opinion editorial, we will examine the background of angiokeratomas, discuss the implications of Palvella’s latest research expansion, and also reflect on how patient privacy and data management play an increasingly important role in modern healthcare.
Understanding Clinically Significant Angiokeratomas
Clinically significant angiokeratomas are superficial vascular malformations of lymphatic origin. They manifest as skin lesions that may cause persistent bleeding, pain, functional impairment, and even a risk of infection. Patients often experience a diminished quality of life due to these troublesome symptoms, and the available treatment options, which are typically procedural interventions, carry a variety of risks such as pain, scarring, and the likelihood of recurrence.
What makes angiokeratomas particularly challenging is the lack of spontaneous regression—they are not inclined to resolve on their own, leaving patients reliant on invasive treatments that are as much about managing an ongoing condition as they are about offering hope for a better quality of life. The current landscape is full of tangled issues when it comes to treating such conditions, and this is where novel therapies like QTORIN rapamycin can potentially create a significant impact.
Diving Into the World of Rare Disease Therapeutics
Palvella’s decision to expand the development of QTORIN rapamycin into this area stems from a growing body of scientific evidence and published case studies indicating potential benefits of off-label rapamycin usage. By choosing to address a rare skin condition that remains untreated by conventional therapies, Palvella is venturing into a domain that is both scientifically intriguing and loaded with challenges.
It is worth noting that advancing treatments for rare diseases often involves navigating a series of tricky parts. This includes the need for intensive clinical trials, relatively small patient populations, and regulatory pathways that can at times seem overwhelming. Despite these hurdles, companies like Palvella persist in their efforts to offer hope where little existed before. In doing so, they are not just working on a drug; they are tackling a complex network of scientific, social, and regulatory challenges.
Balancing Patient Privacy with Cutting-Edge Medical Innovation
While the development of innovative therapies is at the forefront of medical advancement, modern digital practices also dictate that patient trust and privacy must remain a super important part of the equation. Modern healthcare websites manage a wealth of data—ranging from research updates to detailed procedural consent documents—which must be handled responsibly. This requirement is reflected in the detailed cookie consent information seen on platforms like BioSpace.
In many ways, the way healthcare websites use cookies to enhance user interaction has parallels with how physicians and researchers must handle patient data in clinical trials. Both require a transparent, user-centred approach, ensuring that end users—whether patients or website visitors—are fully informed about their rights and the nature of the data being collected.
Learning from Detailed Cookie Consent: Trust and Transparency in Healthcare Websites
The cookie consent details provided by websites like BioSpace serve as a fine example of how transparency can build trust. These details include information about necessary cookies, cookies used for statistical and performance metrics, and those deployed for marketing purposes. By breaking these details down into categories and describing their functions and storage durations, the website provides a clear picture of how user data flows within the digital ecosystem.
For those looking to figure a path through the maze of online data policies, this type of clarity is not only refreshing but also essential. It is a reminder that while medical innovation is critical in the physical realm of treatments, a similar commitment to openness must be applied in the digital realm when it comes to handling personal data.
Using Data Responsibly: An Opinion on Website User Experience and Privacy Protocols
One interesting debate in today’s digital healthcare environment focuses on balancing the fine points of user experience with stringent privacy protocols. While cookies and similar data collection tools are necessary to help websites run smoothly and provide tailored content to users, their management involves several subtle parts that can sometimes seem nerve-racking to the average visitor.
In the case of healthcare journalism, where the stakes include personal health and sensitive research data, ensuring that data is secured responsibly has become a key, yet challenging, pursuit. Using controlled data collection methods not only helps optimize website performance but also builds a trusted relationship with the end-user—whether that individual is a patient seeking health information or a researcher investigating the latest clinical developments.
The Role of Cookies in Enhancing Healthcare Digital Experience
Cookies, when used properly, enable a more efficient and personalized experience for visitors to healthcare websites. Their functions range from maintaining logged-in statuses to tracking user interactions—helping content providers optimize their services in a way that respects user privacy and improves overall satisfaction. Here is a breakdown of why these digital tools are so important:
- Usability Enhancements: Essential cookies help users navigate through secured areas and perform basic functions on the website.
- Performance Metrics: These cookies monitor speed and performance, enabling the continuous improvement of user interfaces and content delivery.
- Security Measures: Cookies like csrfToken play a critical role in preventing attacks and keeping user data safe from malicious interference.
- Personalized Content: Marketing cookies help display more relevant advertisements, which, when managed responsibly, contribute to a tailored user experience.
As the healthcare industry increasingly relies on digital tools to disseminate information and connect with patients, ensuring transparency about data usage is as important as developing cutting-edge therapies to treat diseases. The dual focus on privacy and progress is a reminder that future success in healthcare demands excellence both in science and digital management.
Teaming Up Technology with Medicine: A New Era in Patient Empowerment
The challenges and opportunities that come with developing treatments for rare diseases are closely intertwined with the rapid evolution of digital technology. This new era is marked by efforts to combine data analytics, clinical research, and improved patient interactions in ways that benefit both the healthcare community and the wider public.
As pioneers in the medical field, companies like Palvella Therapeutics are not only developing novel drug therapies for complex conditions but are also harnessing the power of data to improve overall treatment protocols. The focus on detailed consent, both in clinical studies and on healthcare websites, reflects an industry-wide commitment to transparency and ethical data management.
Data Analytics in Healthcare: Benefits, Challenges, and Ethical Considerations
With the vast arrays of data managed today on both clinical and digital fronts, healthcare providers are faced with a host of tricky parts when interpreting and utilizing this information. Data analytics can provide key insights into treatment efficacies, patient responses, and trends that can guide future research and therapies. However, these opportunities come with their tangled issues.
Among these are ensuring that data is collected with the utmost care, stored with robust security protocols, and used in ways that respect patient confidentiality. When data practices are transparent and ethical, they foster a climate of trust—a non-negotiable asset in both clinical research and in managing user experiences on healthcare websites.
The approach to data in healthcare should be seen not as a burden but as a fundamental tool that, when handled correctly, can super charge research and patient care initiatives. A balanced strategy that respects encryption, informed consent, and clear disclosure about data use leads to a digital ecosystem that benefits everyone.
Patient Empowerment: How Informed Consent Fuels Better Care Delivery
In an era where information is abundant, informed consent remains a cornerstone of ethical healthcare practice. Whether it pertains to a complex clinical trial for a rare skin condition or the management of digital cookies on a medical news website, the principle is the same: patients and users have the right to know what data is being collected and how it is used.
This topic can be broken down into several essential segments:
- Clear Communication: Patients should be provided with clear, simple language that outlines how their information is used. Avoiding confusing bits of legal or technical jargon empowers individuals to make informed decisions.
- Right to Modify Preferences: Offering options to customize consent preferences—such as opting out of certain types of data collection—is necessary to honor personal choices in the digital age.
- Ongoing Data Management: Ensuring that consent is not a one-time event but a continuous agreement that adapts as policies change helps maintain a robust sense of trust.
When users feel that they are active participants in managing their own privacy, this level of engagement translates into more robust care delivery and improved patient outcomes. Empowerment through transparency allows digital platforms to act as trusted conduits between complex medical research and everyday users.
The Future of Therapy for Rare Diseases: Challenges and Opportunities
Looking forward, there is no doubt that the road to groundbreaking treatments for rare diseases will be lit by both the relentless pursuit of scientific discovery and a concerted effort to build trust within the digital realm. For conditions like clinically significant angiokeratomas, where current treatment options remain limited, innovative therapies like QTORIN rapamycin present an opportunity to challenge the status quo.
The journey towards regulatory approval and widespread clinical acceptance involves many turns and hurdles. Some of the key points of attention include the careful design and execution of Phase 2 studies, managing the nerve-racking regulatory approvals, and addressing the unpredictable results that early-stage clinical trials often yield.
Weighing the Risk and Rewards: Managing Your Way Through Clinical Trials and Regulatory Hurdles
One of the constant challenges in rare disease research is the balancing act between potential rewards and inherent risks. Clinical trials, by their nature, can be overwhelming and intimidating due to the unpredictable responses and the strict regulatory frameworks that govern them. For a condition as rare and complex as angiokeratomas, even a single misstep in trial design can lead to significant setbacks.
Yet, it is this same rigorous process that ultimately ensures that only safe and effective therapies make it to market. For companies like Palvella, the commitment to working through these tricky parts is a sign of their dedication to delivering meaningful solutions to patients in need. The planned meeting with the FDA and the anticipated initiation of clinical studies later in 2026 are testaments to a methodical approach to treating diseases that are often left in the shadows.
As the landscape of clinical research continues to develop, the need for well-designed, ethically sound trials will only grow more essential. Open communication between regulatory bodies, research institutions, and patient communities is key to steering through the inevitable twists and turns of the approval process.
Public Opinion and Healthcare: Informed Consent in a Digital Age
In today’s interconnected world, where digital interactions are as important as in-person consultations, informed consent extends beyond the clinical environment. Websites and online platforms must also work diligently to keep their audiences informed about their privacy choices and data usage. This level of transparency has become a linchpin in maintaining public trust in healthcare innovations.
The detailed cookie consent information provided by platforms such as BioSpace is a case in point. By showing exactly what data is collected, for how long it is stored, and exactly how it benefits the user experience, healthcare websites demonstrate a commitment to integrity that mirrors the ethical foundations of clinical research. For patients and website visitors alike, this approach offers reassurance that both their personal data and health information are handled with the utmost care.
Conclusion: The Road Ahead in Rare Disease Management and Patient Transparency
As we look to the future, the integration of cutting-edge therapies and robust digital data practices promises a more holistic approach to healthcare. The expansion of Palvella Therapeutics’ QTORIN rapamycin program into clinically significant angiokeratomas highlights the strides that are being made in addressing rare, debilitating skin conditions. At the same time, the detailed emphasis on user privacy and effective data management in digital platforms mirrors the need for transparency in clinical innovations.
Both spheres—scientific research and digital communication—face their own set of tricky parts and tangled issues. In the field of clinical research, every stage of drug development demands careful consideration of safety, efficacy, and ethical responsibility. In the online realm, developers and content managers must figure a path through a delicate balance of enhancing user experience while safeguarding personal data.
This synchronized commitment to excellence is both super important and a must-have for the future of healthcare. Through ethical clinical trials, clear regulatory pathways, and transparent online communication, the journey ahead—although sometimes overwhelming—holds immense promise. In the end, these efforts will not only improve treatments for rare diseases like angiokeratomas but also build a more trusted and empowered relationship between patients, healthcare providers, and the digital platforms that connect them.
As more companies take bold steps in the development of novel therapies and as healthcare websites continue to refine their data practices, the collaborative spirit between innovation and transparency will undoubtedly serve as the cornerstone of progress in the field of medicine. Embracing both the challenges and opportunities of today will fuel the breakthroughs of tomorrow—ensuring that even the most complicated pieces of healthcare research align with modern expectations for privacy, accuracy, and compassion.
In the end, the path forward involves a constant commitment to asking the hard questions: How can we continue to push the boundaries of science while still maintaining an open and honest dialogue with those who entrust us with their care? How can we take advantage of digital tools to enhance, rather than complicate, the patient experience? By working together and fostering a culture of open communication, the multifaceted innovations in both drug development and data management will serve as guiding lights toward a healthier, more informed future.
For patients and stakeholders alike, it is reassuring to know that the pursuit of innovative treatments and transparent practices is a shared journey—a journey in which each step, no matter how nerve-racking or filled with twists and turns, contributes to a brighter, more responsible era in healthcare.
Read more about this topic at
The 25-Year-Quest Behind a Rare Disease Breakthrough
Mayo Clinic researchers blaze a trail of rare disease …