Rethinking Vascular Morphogenesis: A Balancing Act of Mechanical Forces
The latest research on vascular morphogenesis shines new light on how our blood vessels grow and organize themselves. Recent studies have uncovered the critical role that the physical surroundings – particularly the stiffness of the extracellular matrix – play in orchestrating the tricky parts of angiogenic branch elongation and lumen development. This work challenges us to rethink old models by highlighting how pericytes, intraluminal pressure, and biomechanical cues work together in tandem to shape our vasculature.
Understanding the Tricky Parts of Angiogenic Branch Elongation
At the heart of new vessel formation lies the process known as angiogenesis. During this process, endothelial cells (ECs) sprout from existing vessels and migrate collectively to form cord-like structures. As intriguing as these behaviors are, they come with a set of tangled issues that have long perplexed scientists—even when viewed through the lens of cell biology. Researchers now point to the surrounding stiffness of the tissue as a key factor controlling the pace and direction of these movements.
To break it down into simpler terms, think of ECs trying to make their way in an environment that is both supportive and restrictive at the same time—the extracellular matrix (ECM). If the ECM is too soft, the vessels expand in an off-putting and uncoordinated manner, which may disrupt the fine balance between branch elongation and lumen (the central open space) development. On the other hand, when the microenvironment is stiffer, the branches remain narrow, and the ECs can maintain a steady, directional movement. This biomechanical regulation is a game changer in our understanding of how blood vessels are sculpted.
Traditional models have often focused on the biochemical signals—such as vascular endothelial growth factor (VEGF)—leaving the mechanical factors largely in the background. However, emerging evidence shows that the physical forces and the stiffness balance of the ECM are as critical as the chemical cues. When the ECM stiffens locally, it prevents excessive lumen expansion and ensures that the tip ECs keep moving forward at a steady pace. In turn, this sustains proper branch elongation and the formation of a well-organized vascular network.
Perivascular Stiffness and the Role of the Extracellular Matrix
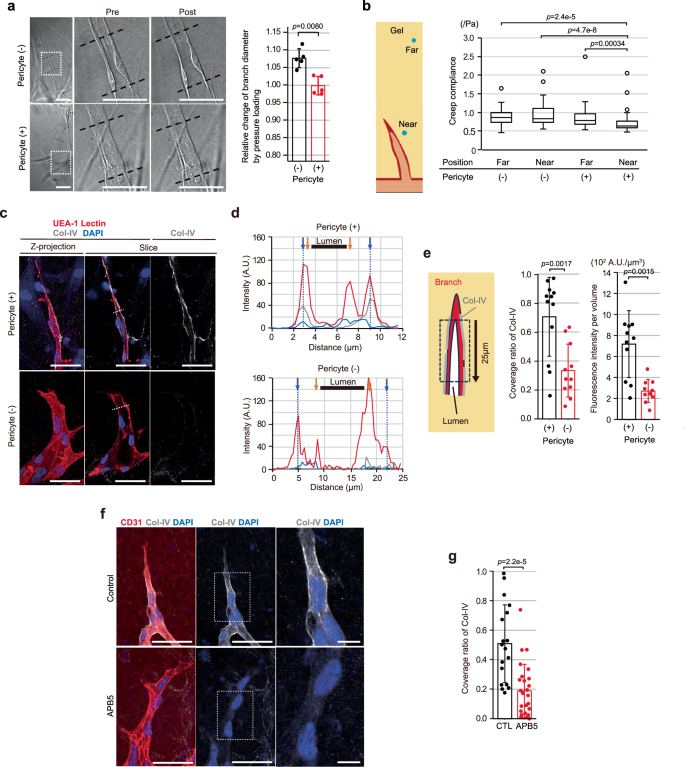
One of the most compelling insights from recent experiments is that mechanical properties of the surrounding ECM directly influence vessel shape and function. Researchers demonstrated that altering the stiffness using agents like transglutaminase (TG) can restrict branch expansion. When such a crosslinker is applied, the vessel walls become less distensible under the influence of intraluminal pressure. This means that instead of having vessels that balloon and lose their coordinated elongation, the ECs are better able to maintain a narrow, elongated branch structure.
In simpler terms, the ECM acts as a kind of scaffold that not only supports but also guides the ECs. This scaffold helps determine just how much a vessel can expand — a factor that can either promote or hinder the forward movement of endothelial tip cells. Consider the ECM as a traffic director. If it signals, “Keep going straight,” the cells form long, narrow vessels. But if its signals are off, the cells might take wrong turns, leading to a messy network of blood vessels.
Key practical takeaways from this work include:
- Regulation by Stiffness: By manipulating the ECM stiffness, scientists can control the rate of both lumen expansion and branch elongation.
- Balancing Intraluminal Pressure: Properly calibrated intraluminal pressure helps in maintaining vessel shape but must be counterbalanced by the ECM’s rigidity.
- Mechanical Integration: The combined mechanical environment—pressure from within and ECM stiffness from without—creates a dynamic force field that determines vessel morphology.
Pericytes: The Unsung Heroes of Vascular Development
While endothelial cells get a lot of attention, pericytes are emerging as key players in managing the mechanical environment needed for effective angiogenesis. Acting as one of the supporting cell types adjacent to ECs, pericytes contribute to the stabilization and maturation of blood vessels. They do this in part by enhancing the deposition of type-IV collagen (Col-IV) onto the vascular basement membrane (VBM). Col-IV deposition is critical to provide structure and stiffness to the growing vessel.
Recent observations indicate that when pericytes are present, the branch diameter remains narrow, and the lumen expansion is kept in check. Without their influence, the delicate balance between branch elongation and lumen development can be disrupted, leading to overly wide or even regressive vessel structures. In a way, pericytes help steer through the complicated pieces of vascular morphogenesis by ensuring that mechanical cues remain balanced.
Specifically, pericytes help manage the following:
- Collagen Deposition: Enhancing Col-IV deposition, which acts as a structural “glue” and stiffening agent on the VBM.
- Maintaining Mechanical Balance: By keeping the vessel narrow, they contribute to the proper directional movement of tip ECs.
- Supporting Vascular Integrity: Their presence ensures that the ECs stay on track and that the tube formation is orderly even when the physical forces are intense.
This naturally raises important questions about how therapies might be designed to influence pericyte activity, especially in situations where vessel formation is off-centered, such as in tumor angiogenesis or in wounds. Understanding this interplay could lead to novel treatments that tweak this mechanical balance for better outcomes.
Intraluminal Pressure: Friend or Foe?
Intraluminal pressure—the force generated by the flow of blood within the vessel—is another key factor that influences vessel formation. Blood flow introduces a certain pressure that can promote the formation of the lumen in the growing vessel. However, if this pressure is too high, it can cause excessive expansion of the lumen which, in turn, interferes with the forward directional movement of the tip ECs.
This phenomenon creates a kind of push-and-pull relationship. On one hand, a certain amount of intraluminal pressure is essential for lumen development. On the other, if that pressure overshoots the desired level in the absence of a sufficiently stiff perivascular environment, the endothelial cells may lose their polarity and directional focus. In other words, the cells lose their way in the messy bits of a dilated and disorganized vessel.
The research illustrates that when external pressure is applied, vessels that are not supported by a stiff ECM or pericytes tend to experience sudden housing expansion and even regression. In contrast, when the ECM stiffness is increased or pericytes are present to mediate vessel wall tension, the intraluminal pressure supports lumen development without impeding branch elongation. This balance is delicate and underscores the need to understand the forces at play when designing clinical interventions.
Important considerations include:
- Controlled Pressure Loading: External modulation of intraluminal pressure in experimental settings shows that excessive pressure impairs branch elongation.
- Rescue via ECM Stiffening: When additional intraluminal pressure is applied, pre-stiffening the ECM can help rescue the forward movement of ECs.
- Clinical Insights: Therapeutic strategies might use this knowledge to modulate intraluminal pressure in pathological states such as tumors or in wound repair.
Mechanical Balance in Vascular Morphogenesis: The Big Picture
The interplay between branch elongation and lumen development in angiogenesis can be summarized as a balancing act, where the fine points are determined by the cooperation between cellular components and their physical environment. On a broader scale, this mechanical balance governs not only the development of blood vessels but might also provide insights into other tissue morphogeneses.
Identifying the physical forces that regulate these processes helps us understand the twists and turns of developmental biology at a more nuanced level. For example, the control of actin polymerization—a process essential for cell movement—is modulated by both biochemical signals and mechanical stresses. Proteins like the F-BAR family and the Arp2/3 complex act almost like sensors, detecting even small differences in membrane tension that occur when a vessel lumen begins to expand. When these proteins are mislocalized due to abnormal mechanical forces, the process of directional movement is disrupted, further skewing the geometry of the vessel.
In practical terms, researchers have observed that when the ECM is stiffened—for instance, by crosslinking agents—these proteins re-establish their proper positioning at the leading edge of ECs. This ensures that tip cells maintain a solid front and continue their directional migration, which is essential for proper branch elongation. This is a clear example of how the once abstract “mechanical balance” plays out in live vascular morphogenesis.
Integrating Biophysical Insights with Clinical Applications
This new perspective on biomechanical regulation has significant implications for the clinical management of various conditions. For instance, in cancer therapy, abnormal vessel formation often leads to inefficient blood flow and can contribute to therapeutic resistance. Tumor vessels tend to be either overly dilated or slightly aberrant due to a disrupted balance between intraluminal pressure and ECM stiffness. Understanding the mechanical cues that guide blood vessel formation could lead to therapies designed to normalize tumor vessels.
Imagine a clinical scenario where a drug is used to modulate ECM stiffness selectively in tumor tissues. Such an approach might help correct the chaotic architecture of the tumor vessels, making them more “normal” and thus more accessible to chemotherapy or targeted agents. Likewise, in wound healing, excessive or insufficient vascular growth can delay proper repair, and adjusting this mechanical balance might expedite tissue regeneration.
Some potential clinical insights drawn from these studies include:
- Tumor Vessel Normalization: Tweaking ECM stiffness or mimicking pericyte functions could normalize blood vessel structure to improve drug delivery.
- Wound Repair Optimization: Adjusting intraluminal pressure and ECM properties might help achieve a more balanced angiogenic response, leading to better healing outcomes.
- Anti-Angiogenic Strategies: In diseases where unwanted vessel growth is a problem, targeting the mechanical forces driving angiogenesis could offer an alternative strategy.
Digging Into the Fine Points: Experimental Insights and Future Directions
Several experiments using microfluidic devices have provided striking visual evidence of the mechanical control over angiogenic morphogenesis. These on-chip assays allow for the state-of-the-art visualization of both endothelial cell movement and the development of the vessel lumen.
One key experiment involved the application of an external hydrostatic pressure on developing vessels. Observations showed that a high pressure load resulted in immediate dilation of the lumen and a sudden drop in the directional migration of the tip cells. However, when the ECM was pre-treated with a stiffening agent, the tip cells kept moving forward, and the lumen size remained nearly constant. This clearly indicates that a stiffer perivascular environment can buffer the intimidating surge of intraluminal pressure, helping the cells sustain their directional movement.
Furthermore, experiments that involved knocking down the expression of COL4A1 and COL4A2 (subunits of type-IV collagen) in ECs resulted in fewer stable vessel formations and even in branch regression. These molecules provide critical support by reinforcing the vascular basement membrane. In the absence of enough Col-IV deposition, the vessels tended to expand excessively and lose the structure needed for efficient branch elongation, underscoring the importance of these proteins in establishing a balanced mechanical field.
Looking at the broader horizon, future research might focus on:
- Mapping Mechanical Signals: Further clarifying the roles of the F-BAR proteins and Arp2/3 complexes as sensors for membrane tension could lead to novel interventions.
- Customizing ECM Stiffness: Developing biomaterials that mimic the mechanical properties of the natural ECM may offer promising targets for regenerative medicine.
- Exploring Pericyte-EC Interactions: Decoding the detailed dialogue between pericytes and ECs will allow us to harness this relationship therapeutically, especially in diseases where angiogenesis is disordered.
Managing Your Way Through the Complicated Pieces of Tissue Engineering
While the idea of controlling vessel growth by tweaking mechanical forces may seem overwhelming at first, the concept suggests a promising direction for tissue engineering. Balancing the internal pressure within vessels against the external stiffness of the ECM represents the nitty-gritty of tissue sculpting. By taking a closer look at these hidden complexities, biomedical engineers can design better scaffolds and materials that mimic the natural environment of blood vessels.
This could involve the use of smart biomaterials—substances designed to change their stiffness in response to environmental cues—in order to adapt dynamically as new vessels form and mature. Such biomaterials would provide the ideal balance, ensuring that ECs continue their directional movement while also forming a properly sized lumen. Imagine a scaffold that gently guides cells to form a long, narrow tube, gradually adjusting its stiffness to suit the evolving needs of the tissue.
In tissue engineering strategies, several approaches are already being explored:
- Hydrogel Engineering: Hydrogels with tunable stiffness offer the flexibility needed to mimic the mechanical environment of the natural ECM.
- Biomimetic Scaffolds: Designs based on natural proteins, such as collagen and fibrin, have been developed to provide both structure and mechanical cues to forming tissues.
- Dynamic Material Adaptation: Future materials might be engineered to change stiffness as cells deposit their own ECM, further harmonizing with the natural process of angiogenesis.
Dealing with the Overwhelming Challenges: A Call for Multidisciplinary Research
One of the reasons the field of angiogenesis has remained so tantalizingly complex is due to the array of intimidating challenges that remain to be solved. The interplay between cell biology and biomechanics encompasses a number of confusing bits—even for seasoned researchers. To fully unlock the secrets of vascular morphogenesis, a broad, multidisciplinary approach is essential.
Collaboration among biologists, engineers, mathematicians, and clinicians is key to resolving the tangled issues presented by the dynamic mechanical forces at play. For instance, biologists provide insights into the subtle parts of cell signaling and protein localization, while engineers can build devices and materials to mimic these conditions precisely. Similarly, mathematicians and computational modelers help simulate these intricate interactions in silico, providing a blueprint for experimental design.
Some steps that can help bridge these disciplines include:
- Integrated Laboratory Approaches: Combining microfluidics, live-cell imaging, and computational modeling to correctly imitate in vivo conditions.
- Collaborative Ventures: Joint projects between tissue engineers and vascular biologists to design biomaterials that match the mechanical profiles of natural ECM.
- Cross-Disciplinary Training: Educating the next generation of researchers to appreciate both the cell-autonomous signals and the mechanical forces that drive tissue morphogenesis.
Emerging Technologies and Future Opportunities
State-of-the-art manufacturing techniques like 3D bioprinting, combined with advanced biomaterials, are providing new avenues for controlling the mechanical environment in tissue engineering. These techniques allow researchers to fabricate scaffolds with precise mechanical gradients that mirror those found in vivo. When applied to vascular tissue engineering, these innovations could lead to the creation of engineered blood vessels that mimic their natural counterparts both functionally and structurally.
Additionally, modern imaging methods, such as high-resolution live-cell microscopy and real-time particle-tracking microrheology, let researchers dive in deeper than ever before into the nitty-gritty of endothelial cell dynamics. With such tools, the moment-to-moment changes in cell behavior in response to mechanical cues can be observed and quantified. This not only enriches our understanding but also empowers us to design interventions that can tweak these responses in a controlled way.
Looking ahead, future research may focus on:
- Personalized Biomaterials: Designing patient-specific scaffolds based on individual ECM profiles for regenerative medicine applications.
- Real-Time Feedback Systems: Developing biosensors that monitor intraluminal pressure and ECM stiffness during vessel formation, allowing for dynamic adjustments.
- Targeted Therapeutics: Using molecular interventions that adjust the localization of key proteins involved in mechanical sensing, such as the F-BAR family and Arp2/3 complexes, to correct aberrant angiogenesis.
Clinical Implications: From Bench to Bedside
The practical implications of these discoveries resonate strongly within the clinical community. For patients with ischemic disorders, chronic wounds, or even diabetic retinopathy, proper vessel formation is critical to restore healthy tissue function. As we begin to understand better how mechanical forces dictate vessel formation, it becomes possible to translate these findings into therapies that manage or even prevent pathological angiogenesis.
One potential clinical strategy involves normalizing the mechanical tension within the tumor microenvironment. Often, tumor vessels are abnormally formed due in large part to a disrupted mechanical balance—the vessels may be too permeable or overly rigid, leading to an inefficient blood supply. By carefully calibrating the ECM stiffness and intraluminal pressure, clinicians might improve drug delivery and even boost the responsiveness of tumors to conventional treatments.
Another promising application lies in wound care. When wounds heal slowly due to poor vascularization, patients suffer from complications that can become nerve-racking. Enhancing localized ECM stiffness or modulating pericyte activity in these areas could promote a more ordered and effective angiogenic response, thus speeding up healing.
In summary, clinical opportunities include:
- Tumor Vasculature Normalization: Regulating ECM stiffness in tumor environments to improve therapeutic outcomes.
- Enhanced Wound Healing: Boosting proper vascular sprouting by fine-tuning local mechanical conditions.
- Management of Ocular Vascular Diseases: Using mechanical cues to correct abnormal blood vessel growth in retina-related disorders.
Conclusion: Steering Through the Twists and Turns of Vascular Formation
In conclusion, the latest research into the biomechanical control of vascular morphogenesis forces us to take a closer look at the hidden complexities of angiogenic growth—those fine points that dictate whether blood vessels will form efficiently or become disordered and dysfunctional. The influence of perivascular stiffness, regulated by the ECM and pericyte activity, is emerging as a key element in this process.
By understanding how intraluminal pressure, ECM stiffness, and protein localization work together to direct the forward movement of tip endothelial cells, we can begin to figure a path toward therapies that not only guide new vessel formation in a controlled way but also correct pathological conditions when the balance is lost. This research sheds light on the intricate yet manageable parts of angiogenesis and urges us to appreciate the dynamic interplay between mechanical forces and cellular behavior.
As we move forward, it will be critical to continue dissecting the complicated pieces of this process. Multidisciplinary research that bridges the gap between cell biology, biomechanics, and material science is indispensable. The emerging technologies of tissue engineering and real-time imaging promise to unlock further secrets behind how blood vessels are built—and how they can be rebuilt when things go awry.
Ultimately, a deep understanding of these mechanisms is not just an academic exercise. It carries super important implications for clinical strategies ranging from cancer treatment to regenerative medicine. By leveraging mechanisms that control the directional spread of endothelial cells, as well as the precise regulation of lumen formation, we have a pathway to designing more effective interventions that target the physical as well as biochemical environment of tissues.
This balanced interplay is much like steering a ship through tricky waters: without a clear understanding of the currents (in this case, mechanical forces), the vessel (our blood vessel) might drift off course. But with continuous feedback from the environment—whether through the stiffness provided by pericytes or the carefully measured intraluminal pressure—the path forward becomes clearer, and the vessel finds its proper way.
It is an exciting time to be working at the intersection of biomechanics and vascular biology. As more researchers dive in and take a closer look at these convincing clues, we can expect to see innovations that not only improve our basic understanding of blood vessel formation but also translate into life-changing therapies for patients facing a litany of vascular-related issues.
While the road ahead is loaded with challenges and intimidating hurdles, the potential rewards are immense. With every new discovery, we gain a more detailed map of the fine shades that determine how our tissues grow and repair themselves. And in that map are the keys to guiding clinical breakthroughs in regenerative medicine, tumor treatment, and beyond.
By embracing a more integrated view—one that accounts for both the chemical signals and the mechanical influences at play in angiogenesis—we can better steer through these twists and turns. This holistic approach promises not only to deepen our comprehension of vascular morphogenesis but also to pave the way for innovative strategies that can ultimately improve patient care.
The future of vascular research is bright, with this paradigm shift urging us to look beyond traditional signaling mechanisms and to recognize the biomechanical orchestra that underpins the formation of life-sustaining blood vessels. Ultimately, the integration of these insights into clinical practice might very well transform the way we manage a host of diseases, ensuring that the delicate balance of life continues to play in harmony.
Originally Post From https://www.nature.com/articles/s41467-025-61804-z
Read more about this topic at
Mechanical Aspects of Angiogenesis – PMC
The role of extracellular matrix in angiogenesis


