Balancing Data Privacy and Healthcare Innovation
In today’s fast-paced digital age, the integration of advanced digital systems with healthcare practices has become both inevitable and transformative. On one side, we have the increasingly complex realm of digital data processing and the crucial consent to cookies policies that enable smooth website operations. On the other side, the medical industry is witnessing groundbreaking innovations, particularly in areas like vascular grafts. This op-ed aims to provide a balanced perspective on these two seemingly distinct yet interconnected topics, while examining the tricky parts of data privacy as well as the exciting advances in medical device technology.
Understanding Digital Consent on Healthcare Platforms
Digital consent forms, such as cookie notifications, are a fundamental element of modern online platforms. They are necessary not only for ensuring proper functionality but also for integrating third-party content, statistical analysis, and personalized advertising. Many users may find it a bit intimidating when faced with a pop-up asking for consent to processing personal device information. However, beyond the nerve-racking legal lingo lie processes that enable websites to improve user experience and preserve functionality.
Decoding the Cookie Consent Process
When you click the “Accept All” button on a website, you are essentially agreeing to the transfer of data to various third parties, sometimes even beyond national borders. This includes sophisticated services like Google Analytics, which appropriates only limited bits of information to select advertising targets and measure the success of content strategies. In contrast, choosing the “Reject All” function restricts the website to setting only essential cookies necessary for basic site operation.
There are several subtle details involved in these settings, such as:
- The integration of external services and social media elements.
- Statistical analysis to develop personalized user experiences.
- Trans-border data transfer, which sometimes means that data might be accessible to authorities in other countries.
Although the cookie consent process may seem tangled with tricky parts, it is worth noting that robust privacy policies and clear usage instructions are designed to help users steer through these confusing bits with ease. Users always have the freedom to change their preferences later via the advanced settings located at the bottom of many websites.
Vascular Grafts: A Key Innovation in Modern Medicine
While digital consent processes continue to evolve, the healthcare industry is also steering through its own set of complicated pieces. Among the most promising advancements is the development and utilization of vascular grafts. These medical devices play an essential role in managing cardiovascular conditions by replacing, repairing, or bypassing damaged blood vessels. A closer look into their development reveals an exciting blend of advanced technology and improved surgical techniques that promise to revolutionize patient outcomes.
The Role of Vascular Grafts in Cardiovascular Health
Vascular grafts have made a significant impact on modern surgical practices. They serve as artificial or biological conduits intended for patients dealing with conditions such as atherosclerosis, aneurysms, or vascular injuries. Created either from synthetic materials like Dacron or ePTFE or derived from biological tissues from human or animal sources, these grafts ensure durable and efficient blood flow. This is especially important for patients undergoing coronary artery bypass surgery, hemodialysis, and peripheral artery reconstruction.
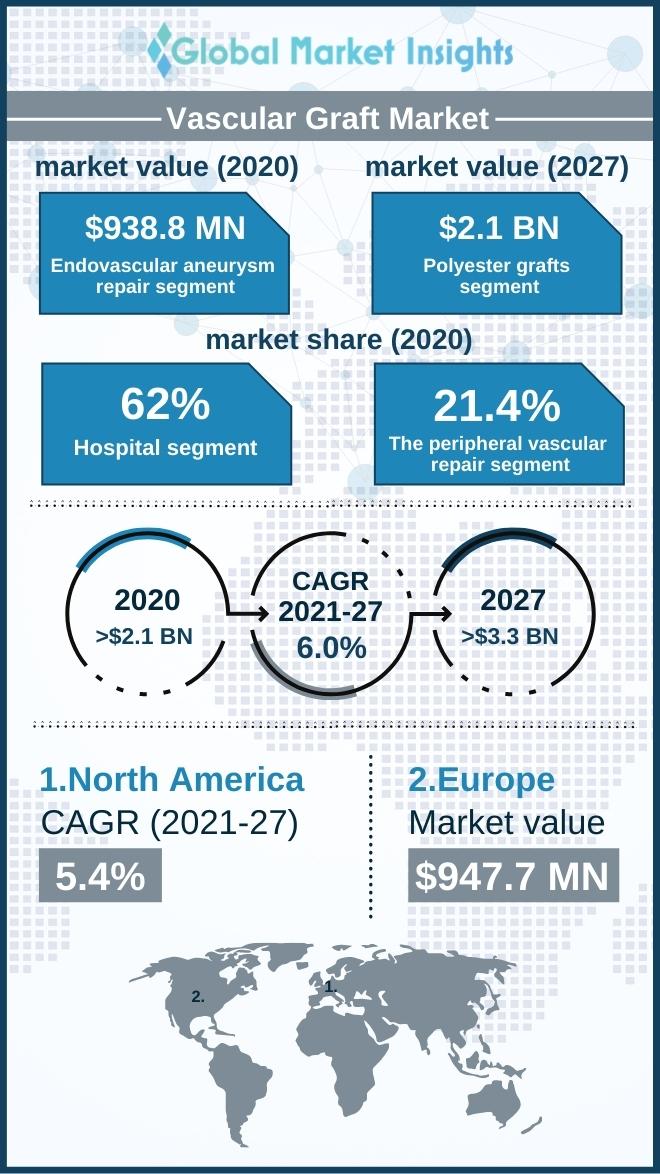
The global vascular graft market is on a strong upward trajectory. According to recent analyses, the market size was estimated at approximately USD 1,863.09 million in 2024 and is projected to expand at a CAGR of 6.86% from 2025 to 2032, reaching an anticipated USD 3,153.39 million by the end of 2032. This growth is driven by the increasing prevalence of cardiovascular disorders and the rising awareness of early diagnosis, which in turn necessitates timely treatment and surgical interventions.
Analyzing the Expanding Global Market for Vascular Grafts
The expanding vascular graft market is a testament to the intertwined progress of modern medicine and innovative surgical technology. A closer look at this industry reveals several layers of complexity, with each layer offering its own set of critical challenges and opportunities for the stakeholders involved.
Market Dynamics and Growth Drivers
There are a number of factors fueling the rapid growth of the vascular grafts market:
- Increased Prevalence of Cardiovascular Diseases: With rates of hypertension, diabetes, and obesity rising worldwide, the incidence of cardiovascular disorders is also on the upswing. This drives a higher demand for surgical interventions that require vascular grafts.
- Early Diagnosis and Screening Programs: Enhanced awareness and improved screening protocols are enabling earlier diagnosis of conditions such as atherosclerosis and aneurysms. Early detection is essential for successful and timely treatments, further underpinning the need for vascular grafts.
- Technological Advances in Surgical Techniques: Modern surgical technologies, such as endovascular repair procedures, have not only made surgeries less invasive but have also improved recovery times and patient outcomes.
- Robust Healthcare Infrastructure: Particularly in regions like North America, high healthcare expenditure combined with advanced medical facilities provides an ideal environment for the adoption of these innovative grafts.
While these drivers contribute to market growth, they also introduce some confusing bits, especially in the realm of regulatory compliance and cross-border data sharing associated with digital health tools. The connection between data analytics related to patient outcomes and regional market strategies often necessitates a careful balancing act between innovation and patient privacy.
Global Regional Dominance: North America Leading the Way
Geographically, North America stands out as the dominant player in the vascular graft market. This leadership is attributed to multiple factors such as a high prevalence of vascular diseases, a well-established healthcare system, and the availability of advanced surgical technologies. The region is also home to several major medical device companies, which continuously push the frontier of innovation with new and improved vascular graft products.
In addition to these factors, North America benefits from favorable reimbursement policies and high healthcare expenditure. This environment has paved the way for complex surgical procedures and has encouraged more aggressive product developments in the vascular graft space. The regional industry’s continuous efforts to break down barriers and tackle challenging issues have set an excellent example for global markets.
Exploring Innovative Surgical Devices and Techniques
Innovation in the vascular grafts market is not just about new products; it’s about introducing devices that significantly improve patient outcomes. Companies across the globe are investing in state-of-the-art technologies to enhance the effectiveness of surgical procedures.
Recent Medical Device Innovations and FDA Approvals
Several key developments in recent years highlight the rapid evolution of vascular graft technology:
- Breakthrough Designations: In November 2024, Healionics received the FDA Breakthrough Device designation for its StargraftTM arteriovenous graft. This innovative device is designed specifically to improve outcomes in dialysis access, by significantly reducing infection and thrombosis risks.
- First-of-its-Kind Endovascular Grafts: In January 2024, W. L. Gore & Associates achieved FDA approval for the GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis (TAMBE). This is the first off-the-shelf endovascular graft aimed at treating complex aneurysmal disease involving the visceral aorta.
These approvals reflect the commitment of industry leaders to address the tricky parts connected with advanced surgical instruments and open up avenues for safer and more reliable treatment options. Each innovation further strengthens the market position and is expected to accelerate growth in the coming years.
Technological Integration into Surgical Procedures
The adoption of integrated surgical devices has helped healthcare professionals find their way through the challenging maze of cardiovascular treatments. For instance, companies such as Medtronic and Gore Medical have built robust portfolios of vascular grafts tailored for specific conditions. Medtronic’s EndurantTM II Stent Graft System, for example, offers enhanced conformability and user-friendliness for abdominal aortic aneurysm repairs, while Gore Medical’s GORE® EXCLUDER® AAA Endoprosthesis is celebrated for its long-term performance and reliability.
To clarify the state-of-the-art landscape in surgical device technology, consider the following table:
| Company | Device Name | Key Feature | Target Procedure |
|---|---|---|---|
| Medtronic | EndurantTM II Stent Graft System | Enhanced Conformability & Usability | Abdominal Aortic Aneurysm Repair |
| Gore Medical | GORE® EXCLUDER® AAA Endoprosthesis | Reliable Long-Term Performance | Coronary and Peripheral Artery Reconstruction |
| Terumo India | TREO® Stent-Graft System | Dual Fixation (Suprarenal and Infrarenal) | Endovascular Aneurysm Repair (EVAR) |
This table not only provides a snapshot of the current state of innovation but also highlights the rich tapestry of options available that help surgeons manage the little twists of complex cardiovascular conditions.
Challenges and Opportunities for Vascular Graft Market Players
Although the vascular graft market offers promising opportunities, it is also loaded with issues that require careful planning and mitigation. Companies operating in this realm often face a range of challenging parts—from regulatory hurdles and intellectual property concerns to market competition and pricing pressures. These tricky parts demand that companies continuously engage in product development, research, and clinical validation to maintain their competitive edge.
Regulatory and Compliance Considerations
One of the nerve-racking aspects of developing new medical devices is dealing with the regulatory landscape. Approval processes like those mandated by the FDA are both essential and demanding, requiring thorough clinical trials and robust documentation. Given that many of these procedures involve sensitive patient data, manufacturers also have to meticulously manage data privacy and security issues when collaborating with third-party analytics services.
Key strategies used by companies include:
- Robust Clinical Trials: Rigorous testing regimes to ensure safety and performance.
- Transparent Data Policies: Clear consent frameworks for patient data usage, analogous to cookie consent protocols in the digital world.
- Cross-Border Compliance: Adapting to various regulatory requirements in different global markets.
These careful measures allow medical device providers to not only meet but often exceed regulatory standards, despite the overwhelming obstacles posed by the constantly shifting compliance requirements.
Innovation and Market Competition
The competitive landscape within the vascular graft market is increasingly intense. Key players—ranging from BD, Medtronic, W. L. Gore & Associates, to emerging companies like Healionics—are all striving to gain a larger market share by continually introducing new product lines and improving existing ones. Unquestionably, successful companies are those that can successfully balance technological innovation with strategic marketing and robust after-sales support.
Some of the strategies that companies are adopting include:
- Product Portfolio Expansion: Diversifying offerings to address various vascular conditions.
- Collaborative Research: Partnering with academic institutions and research centers to share the nitty-gritty of clinical data.
- User-Centric Design: Focusing on the practical needs of surgeons and patients by introducing devices that are both intuitive and effective.
These approaches help companies not only manage the tangled issues of technological innovation but also position themselves as trendsetters in an industry that is as competitive as it is dynamic.
Integrating Digital Analytics and Medical Advancements
There is an intriguing intersection between digital data processing and the world of medical innovation. Like the cookie consent processes that ensure transparent data usage on websites, the healthcare industry relies on similar principles to protect patient data while driving clinical research and innovation. Advanced data analytics, much like Google Analytics for digital platforms, are now essential in shaping clinical outcomes and product development strategies in the medical devices sector.
Leveraging Data for Improved Patient Outcomes
Data collected from clinical trials, postoperative monitoring, and even patient feedback is used to refine existing devices and develop new technologies. This process requires careful management of what might be seen as subtle details within large datasets. The following points illustrate how data analytics is critical in this field:
- Customized Treatment Approaches: By analyzing patient data, healthcare professionals can offer more personalized treatment plans tailored to individual needs.
- Predictive Modeling: Using historical data to forecast potential complications and intervene early.
- Continuous Product Improvement: Data-driven insights facilitate rapid improvements in device performance and safety.
Although collecting and processing such detailed data can be overwhelming, the resulting insights prove invaluable in saving lives and improving the quality of life for patients requiring vascular interventions.
Looking Ahead: The Future of Vascular Grafts and Digital Privacy
In wrapping up our exploration of both digital consent in healthcare platforms and the rapidly evolving vascular graft market, it becomes clear that the future of medicine is intertwined with advanced technology. The digital demands of today, such as managing confusing bits in data privacy protocols, mirror the innovative strides being made in surgical devices and cardiovascular treatments.
Future Trends in Vascular Graft Technology
The vascular graft market is expected to grow significantly, driven by several key trends, including:
- Advanced Material Science: Continued research into synthetic and biological materials that boost graft longevity and biocompatibility.
- Minimally Invasive Techniques: The rise of endovascular procedures that minimize patient recovery time and lower surgical risks.
- Customization and 3D Printing: Personalized grafts created on-demand using cutting-edge 3D printing technology.
- Global Expansion: Increased accessibility in emerging markets where cardiovascular diseases are on the rise due to changing lifestyles and aging populations.
These innovations are set to redefine the fine points of vascular surgery, offering hope to patients worldwide and intensifying competition among companies dedicated to enhancing patient care.
Balancing Privacy with Progress
As we continue to witness rapid changes in healthcare, ensuring that our data privacy practices keep pace is equally critical. Institutions must figure a path that enables them to harness the power of digital analytics while safeguarding patient data with security protocols that are as rigorous as those applied in today’s digital world.
The challenges remain, but with robust policy frameworks, modular data controls, and an unwavering commitment to patient welfare, the journey ahead is both promising and full of potential. Whether it is in the realm of technological integrations on websites or in the delivery of essential medical treatments through advanced devices, the twists and turns of the future should be viewed as opportunities to continuously improve and innovate.
Conclusion: Embracing a Future of Innovation and Responsibility
The intertwined world of digital data management and advanced healthcare innovation demonstrates that progress often comes coupled with challenging parts. While the cookie consent process might seem loaded with legal jargon and complicated pieces, it ultimately functions to protect user privacy and ensure compliance in the digital environment. Similarly, the burgeoning vascular graft market—driven by innovative surgical devices, increasing disease prevalence, and advanced healthcare infrastructures—illustrates the exciting, sometimes intimidating, journey modern medicine is undertaking.
This op-ed has taken a closer look at the fine points and subtle parts of two vital areas: the integration of digital consent mechanisms in healthcare websites and the dynamic evolution of the vascular grafts market. By exploring both, we can appreciate the importance of balancing rigorous data privacy with the relentless push for improved patient outcomes through technological innovation.
For healthcare providers, regulators, and technology companies alike, the urgent call is to continuously work through these tangled issues. This means fostering environments where digital advances coexist harmoniously with patient safety, and where market innovations are matched by strong data protection policies.
Ultimately, the future of healthcare will depend on our ability to take a closer look at both the digital and medical realms, understanding that the challenges are as much about solving the tricky parts of regulation and data management as they are about developing life-saving advances in treatment options. It is a future where every new device, every updated privacy policy, and every fresh approach to data analytics plays a critical role in ensuring not only technological progress but also the well-being and trust of the patients we serve.
As our society continues to evolve, embracing both digital responsibility and clinical innovation will remain a must-have principle. Whether you’re a patient, a healthcare provider, or a technology enthusiast, keeping abreast of these ever-changing landscapes is super important. It is a charged time full of promise—a time in which we must be prepared to figure a path through both the nerve-racking complexities of digital data management and the exciting advances in medical technology.
In this vein, industry leaders are encouraged to continue investing in research, refining their technological tools, and maintaining open lines of communication with regulatory bodies. This approach will help ensure that every stakeholder—from manufacturers to end-users—navigates the charged world of modern medicine with confidence and clarity.
The advances in vascular graft technology, the meticulous designs of surgical devices, and the seamless integration of digital analytics all point to one undeniable fact: the future of healthcare is bright, and with a balanced focus on innovation and responsibility, we are well equipped to meet the challenges and opportunities of tomorrow.
In conclusion, whether you are diving into the detailed world of digital consent settings on a healthcare website, or figuring a path through the rapid advancements of the vascular graft market, remember that every step toward progress is a step toward a safer, more efficient, and more responsive healthcare system. The journey may be full of twists and turns, but with clear vision, patient care remains at the heart of all developments.
Originally Post From https://www.openpr.com/news/4238683/global-vascular-grafts-market-to-reach-usd-3-153-39-million
Read more about this topic at
Promoting blood vessel growth in ischemic diseases
Accelerating vascular graft development: Adipose-derived …