Innovating Stroke Therapy: A New Chapter in UV Laser Technology
The announcement of a strategic collaboration between EndoUVtech and CeramOptec has sparked many conversations in the medical community. As a professional in the field of healthcare journalism, I find it fascinating to see how industries converge to address challenging issues in neurovascular care. This partnership represents a significant turning point in the development of UV laser therapies that target residual blood clots in post-thrombectomy stroke patients. In this opinion editorial, I will take a closer look at the various components of this innovation, contemplating both its promising benefits and the tricky parts it must overcome.
UV Laser Stroke Therapy: A Promising Alternative to Traditional Methods
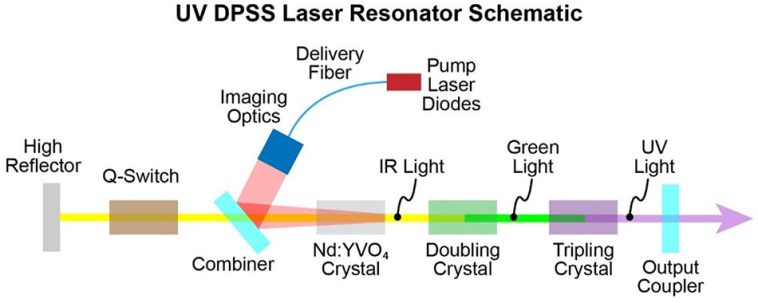
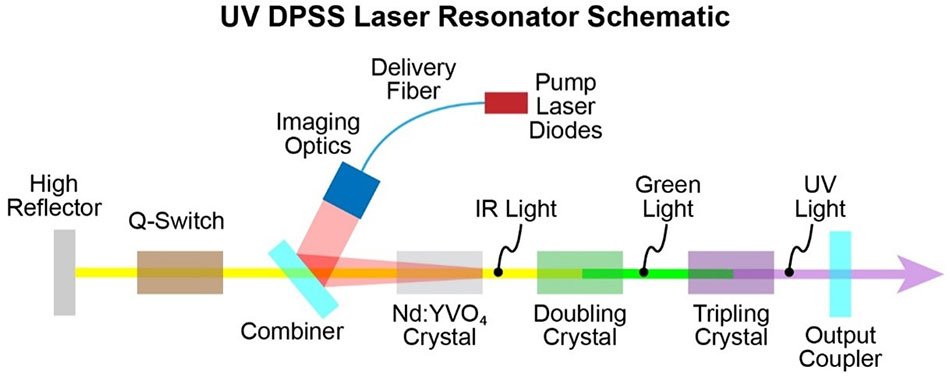
Traditional stroke treatments have long been limited in their approach to revascularization and clot removal. Existing procedures generally focus on large vessel occlusions; however, they may miss the fragmented clots that remain hidden in smaller cerebral vessels. EndoUVtech’s approach uses a unique UV laser system, delivered by a custom fiber optic cable and balloon catheter combination, to safely dissolve these complicated pieces of residual clots.
The beauty of the system lies in its ability to trigger a natural photochemical reaction within the brain’s vessels. This reaction, activated by the precise delivery of UV energy, breaks down the dangerous platelet clots that often cause additional complications. With such an innovation, clinicians might soon have more effective tools to reduce the nerve-racking risk of long-term disabilities in stroke survivors.
Key Benefits of UV Laser Technology in Stroke Care
One of the most exciting aspects of EndoUVtech’s breakthrough lies in the device’s promise to improve patient outcomes by offering controlled arterial dilation while minimizing vessel trauma. By integrating UV laser technology into post-procedural care, the system is designed to reach otherwise inaccessible clots, thereby promoting more complete revascularization. In this way, it tackles a persistent clinical gap left by current treatment options.
In summary, some of the key benefits include:
- Enhanced clot dissolution through a photochemical reaction
- Improved safety profile by reducing excess vessel trauma
- Potential reduction in secondary strokes and long-term disability risk
- An add-on solution that complements current revascularization techniques
Advanced Optical Fibre Manufacturing: The Role of CeramOptec
CeramOptec’s involvement in this collaboration cannot be overstated. As a global leader in specialty optical fibers, the company’s advanced manufacturing capabilities have proven to be essential for the commercialization of EndoUVtech’s device. The custom fiber they produce is thinner than a human hair, a feature that facilitates an accurate delivery of UV energy deep into the cerebral vessels.
Some of the impressive aspects of CeramOptec’s fiber technology include:
- Precision manufacturing that guarantees consistent performance
- Scalable production capabilities to support rapid market expansion
- The adaptability required to meet strict medical device standards
This precision is critical to ensuring that the UV laser system can effectively get around the tiny, yet troublesome residual clots that standard surgical techniques might miss. With CeramOptec’s support, EndoUVtech is well-equipped to face the twisting turns of medical device production and meet the regulatory demands ahead.
The FDA Submission: Approaching Regulatory Milestones
One of the main milestones on the horizon for EndoUVtech is its upcoming submission to the U.S. Food and Drug Administration (FDA), targeted for Q4 2025. The process of navigating through the FDA’s rigorous testing and approval channels is known to be scary and loaded with problems. However, the potential benefits that accompany this innovative UV laser technology make the task well worth the effort.
Approaching regulatory approval involves a systematic approach to ensure that every tricky part of the device’s functionality is studied meticulously. Key considerations include:
- Safety: How will the laser system interact with human tissue? A series of tests are needed to ensure there is no unintended damage.
- Efficacy: Can the device reliably dissolve even the most subtle parts of residual clots? Clinical trials must dig into these details.
- Reproducibility: Will every unit of the device perform consistently under similar conditions?
Clinical trials are predisposed to many nerve-racking twists and turns. Yet, the promise of improved patient recovery outcomes and a safer, more controlled treatment mechanism provides strong motivation for regulatory agencies to give this new therapy serious consideration.
Understanding the Photochemical Process in UV Laser Therapy
At the core of EndoUVtech’s innovation is a complex biochemical process triggered by UV exposure. When UV light is precisely applied to targeted areas in the brain, it ignites a photochemical reaction that helps break down the fibrin structure of the clot. This ability to use light to induce a chemical change is not completely new in the world of medicine, but its application in stroke care is particularly promising.
The photochemical process includes several key steps:
- Laser Delivery: A thin optical fiber, capable of delivering focused UV energy, is inserted via a catheter in conjunction with a balloon. This combination ensures the precise positioning of the laser.
- Photoactivation: Upon exposure, certain molecules within the clot absorb the UV energy, leading to a reaction that splits the clot into smaller, more manageable pieces.
- Enhanced Revascularization: With the assistance of mechanical thrombectomy, the dissolved clots can be more easily cleared, promoting the return of blood flow and reducing the risk of further damage.
This method is not without its tangled issues. The interplay between UV light and biological tissue can sometimes be unpredictable, and ensuring that the laser energy is delivered with pinpoint precision is essential. Fortunately, the collaborative expertise of engineers, clinicians, and manufacturing specialists is helping to figure a path through these challenges.
The Promise of Improved Neurovascular Outcomes
One of the most compelling reasons for the development of this UV laser-based therapy is its potential to enhance outcomes for stroke survivors. Traditional interventions often leave patients at risk for secondary strokes due to chewed-up residual clots that many times remain after the procedure. With the integration of UV laser treatment, clinicians may be better equipped to break down these residual clots, leading to a more complete revascularization process.
This novel approach can lead to:
- A reduction in follow-up procedures
- Lower risk of post-procedural complications
- Improved recovery times for patients
- A decreased likelihood of long-term neurological deficits
For many patients and caregivers, the potential of such an improved neurovascular outcome is especially appealing, given the nerve-racking aftermath that many stroke survivors face. This technology not only addresses immediate clinical needs but also holds the promise of transforming long-term recovery and rehabilitation strategies.
Overcoming the Tricky Parts in Medical Device Commercialization
Nevertheless, the journey from laboratory innovation to full-scale commercialization is loaded with several challenging twists and turns. Developing a new medical device involves more than just fine-tuning its functionality; it requires careful strategic planning in production, distribution, and market adoption.
Some of the most intimidating aspects include:
- Scalability: The ability of CeramOptec to manufacture the high-precision optical fibers in large volumes is a must-have element for this transition.
- Cost-effectiveness: Balancing the cutting-edge technology with cost constraints to keep the product accessible in a competitive market.
- Regulatory hurdles: Meeting stringent standards set by authorities worldwide, which often involve diligent testing and repeated assessments.
- Market education: Clinicians must be convinced of the device’s efficacy and trained on its proper use. This process is critical to ensure widespread adaptation.
EndoUVtech and CeramOptec’s partnership represents a strategic alliance where the strengths of each player are utilized to find your way through these inherent challenges. By leveraging CeramOptec’s advanced fiber manufacturing and EndoUVtech’s innovative treatment approach, the companies aim to streamline the process of bringing this potentially transformative therapy to market.
The Strategic Role of Collaborations in Healthcare Innovation
Partnerships in healthcare are not new, but they have taken on increased importance as innovation grows increasingly complex and regulated. Collaborations like the one between EndoUVtech and CeramOptec serve as prime examples of how industry players can manage their way through complicated pieces and work together effectively.
This alliance is a model for industry collaborations because it demonstrates several important points:
| Aspect | How the Partnership Addresses It |
|---|---|
| Innovation |
|
| Production Scalability |
|
| Market Acceptance |
|
This table highlights the various facets that are being addressed by pooling industry expertise. Such collaborations are not just beneficial but absolutely necessary in today’s environment where every innovation is intertwined with regulatory, technical, and market-related challenges.
Examining the Long-Term Outlook for UV Laser Stroke Therapy
While the current progress made by EndoUVtech and CeramOptec is commendable, it is important to consider what the future may hold if their advancements prove to be clinically effective and commercially viable. The long-term outlook for UV laser stroke therapy is filled with possibilities, but it also calls for cautious optimism as more research and real-world data are needed.
In the coming years, successful FDA submissions and subsequent clinical adoption could pave the way for:
- Broader acceptance and implementation of UV-based therapies across multiple vascular conditions
- Further refinement of the technology to address other challenging aspects of endovascular care
- Enhanced training programs for clinicians, ensuring that the new techniques are properly integrated into existing stroke treatment protocols
- Increased investment and research into photochemical applications for other areas of medical intervention
The development of UV laser technology marks a key turning point in stroke therapy and may prompt an evolution in our overall approach to treating systemic circulatory issues.
Looking at the Bigger Picture: The Intersection of Technology and Patient Care
What stands out most in this evolution is the overall trend towards merging high-precision technology with patient-centered care. Innovations like the EndoSmartUV laser system are a testament to the need for solutions that do more than merely treat symptoms—they address the underlying causes of medical issues.
This evolution is being driven by several factors:
- Technological Advancements: With increasingly refined laser tools and optical fibers, the potential to target microscopic structures in the human body is now within reach.
- Collaborative Networks: Cross-industry partnerships bring together experts from diverse fields, ensuring that each perspective is taken into account when developing new therapies.
- Patient Demand: Today’s patient is well-informed and expects therapies that not only promise quick fixes but also provide long-term health benefits with minimal side effects.
This intersection of technology and compassionate healthcare is precisely what modern medicine needs in an era dealing with challenging bits of legacy treatment methods. It reminds us that while the road to progress is often intimidating and full of small distinctions, the journey continues because the potential for improved patient outcomes is too significant to ignore.
Challenges and Considerations: A Realistic Look Ahead
While the promise of UV laser stroke therapy is exhilarating, it is important to recognize that the pathway forward is dotted with potential obstacles. Many of the challenges relate to the multifaceted nature of medical innovation, where every advancement comes with its own set of tangled issues.
Among these challenges are:
- Technical Optimization: Fine-tuning the interaction between UV energy and brain tissue is key. Even the smallest miscalculation can lead to unintended tissue damage, making rigorous testing essential.
- Regulatory Navigation: Dealing with compliance means that new technologies must meet exceptionally strict standards across diverse markets—a process that is both time-consuming and overwhelming.
- Market Dynamics: Healthcare providers need robust evidence of safety and efficacy before adopting new technologies. Scientists and engineers alike must work to generate clinical data that demonstrates clear advantages over current methodologies.
- Investment in Education: Training and educating clinicians on the proper usage of these advanced tools is crucial. Without a clear understanding of how to integrate new technology into everyday practices, even the best innovations might face slow uptake.
Addressing these challenges will involve a concerted effort from all stakeholders involved, from device manufacturers to regulatory bodies, and most importantly, the healthcare providers who will ultimately be using these devices in clinical settings.
Weighing In: Is the UV Laser Approach the Future of Stroke Treatment?
In my view, the strategic partnership between EndoUVtech and CeramOptec exemplifies the kind of forward-thinking innovation necessary to disrupt the traditional models of stroke treatment. The combination of UV laser technology and advanced optical fibers provides a compelling case for renewed optimism in stroke recovery, especially for those patients who have previously faced life-altering complications following conventional procedures.
The potential benefits speak for themselves:
- Enhanced ability to remove residual clots
- Potential reduction in long-term neurological deficits
- Improved quality of life for stroke survivors
- A new avenue for addressing strokes that are complicated by small, residual blockages
However, it is also necessary to acknowledge that every breakthrough in medical technology is accompanied by intense scrutiny and the need for robust validation. The inclusion of UV energy must be thoroughly understood, and the dosage precisely administered, to avoid the nerve-racking possibility of unintentional side effects.
Clinical Trials and the Road to Real-World Validation
As EndoUVtech prepares for its FDA submission, it also embarks on a journey that will involve comprehensive clinical trials. These studies are critical not only for regulatory validation but also for building clinicians’ confidence. Real-world data is key to ascertaining the device’s capability in managing the confused bits of residual clots safely and efficiently.
Important stages in the clinical pipeline are likely to include:
- Initial safety trials to determine the appropriate UV energy levels that can be safely administered.
- Efficacy studies that compare traditional clot removal techniques with the new UV laser approach.
- Long-term follow-ups that document stroke survivors’ quality of life, highlighting improvements in neurological outcomes.
- Comparative studies that differentiate outcomes based on various patient demographics and conditions.
With rigorous clinical trials, stakeholders will be better able to work through the nerve-racking twists and turns of bringing a new technology to market. The resulting confidence in the measured safety and efficacy of the EndoSmartUV system could, in a not-so-distant future, redefine the standard of care for patients with ischemic strokes.
Patient-Centered Outcomes: The End Goal of Innovation
At the heart of every medical innovation is the patient. The ultimate measure of success for any new therapy is its ability to improve patient outcomes in a safe and dependable manner. For stroke survivors, the promise of a treatment that can dissolve residual clots while minimizing tissue damage stands to significantly improve day-to-day living.
Some of the expected outcomes for patients include:
- Faster Recovery Times: With more effective clot dissolution, patients could see a more rapid return to their daily routines.
- Decreased Hospital Stays: Reduced complications might translate into shorter hospitalization periods.
- Lower Risk of Secondary Events: By targeting clots that other therapies might leave behind, patients may enjoy longer-term health benefits.
- Improved Quality of Life: Fewer neurological impairments mean better overall physical and mental well-being.
In essence, the true test of EndoUVtech’s UV laser technology will be measured by its success at transforming the landscape of stroke therapy through these patient-centered improvements.
Pondering the Future: Broader Implications in Medical Device Innovations
The current advancement in UV laser technology for stroke therapy might just be the tip of the iceberg. As we foster relationships between technology companies and healthcare providers, new pathways for innovation may emerge in related fields, including cardiovascular care and even alternative medical treatments. The cross-disciplinary nature of these innovations highlights the importance of stepping beyond traditional silos and taking a closer look at how various fields can complement one another.
Looking ahead, the broader implications include:
- Advances in Optical Technologies: Enhanced laser and fiber optics could find applications in other areas such as oncology, dermatology, and minimally invasive surgeries.
- Expanded Therapeutic Options: By integrating photochemical methods, researchers might develop alternative therapies for conditions that have, until now, been managed by more invasive means.
- Collaborative Ecosystems: The success of partnerships like that of EndoUVtech and CeramOptec can serve as a blueprint for future collaborations between tech innovators and medical device manufacturers.
This broader view underscores the need to dig into every fine point of inter-industry collaboration. As these relationships evolve, they hold the promise of radically reshaping the delivery of healthcare, paving the way for safer, more effective treatments that can improve lives on a global scale.
Embracing the Complexity: Managing Your Way Through Medical Innovation
Every new medical breakthrough comes wrapped in a bundle of complicated pieces. While the science behind EndoUVtech’s UV laser system is exciting, it is also filled with subtle parts that need careful study, from the precise calibration of the UV light to the delicate interplay of photochemical reactions. As professionals in the field, it is our duty to acknowledge the tangled issues, understand the underlying physiology, and engage in informed debates about the potential impact on clinical practice.
This process involves several essential steps:
- Rigorous Research: Academics and industry experts must continuously study the effects of UV lasers in brain tissues, ensuring that the technology does not inadvertently cause harm.
- Interdisciplinary Collaboration: Doctors, engineers, and regulatory specialists need to work together to figure a path that balances innovation with patient safety.
- Transparent Reporting: The success of clinical trials and ongoing studies should be communicated transparently, so that both the medical community and patients understand the benefits and risks involved.
Embracing the complexity of such innovation means that, as the industry digs into its hidden challenges, each small twist is addressed with diligence and care. Ultimately, this approach will ensure that the final product not only meets regulatory standards but also offers a reliably safe therapeutic option for stroke patients worldwide.
Final Thoughts: Charting a Bold New Course in Stroke Treatment
In closing, the strategic partnership between EndoUVtech and CeramOptec is more than just a business collaboration—it represents a bold step toward transforming the way we treat strokes. By integrating UV laser technology with specialized optical fibers, the companies are taking a significant leap into a future where precision, safety, and improved patient outcomes combine to redefine stroke therapy.
While there are undoubtedly many nerve-racking and overwhelming challenges to get around, including scaling production, meeting strict regulatory criteria, and ensuring consistent clinical success, the collective drive to innovate is clear. The new UV laser approach not only promises to address the mismatches in current treatment protocols but also stretches the boundaries of what we thought possible in neurovascular care.
As the medical community watches closely, there remains a cautious optimism. We find ourselves at the intersection of technology and compassionate care—a place where advanced optical manufacturing, refined clinical methods, and patient safety converge to create tangible improvements in health outcomes.
Ultimately, the ongoing evolution in stroke treatment reminds us that progress is often achieved by facing the tricky parts head-on. Whether it’s untangling the nitty-gritty of photochemical reactions or working through the nerve-racking process of FDA submissions, every step brings us closer to a future where medical innovation leads to healthier, more resilient communities.
A Vision for the Future: Integrating Innovative Technologies into Everyday Medicine
The tale of EndoUVtech and CeramOptec reflects a larger trend in modern medicine—one where boundaries between disciplines blur, allowing experts to take a closer look at systems that have long been taken for granted. In this scenario, innovative technologies such as UV laser treatments are not merely incremental improvements; they represent a fundamental shift towards more precise, patient-friendly approaches to care.
In the near future, we can envision:
- The integration of similar laser-based technologies in less invasive surgeries, which could revolutionize treatment options for various ailments.
- Customized treatment regimes where the dosage and duration of UV exposure are perfectly tailored to individual patient needs, reducing the risk of secondary complications.
- Expanded collaboration across research labs globally, pooling data and insights to streamline the development cycle for new therapies.
- A more informed patient population, empowered by transparent clinical data that demystifies the complex dance of light and biology at work in new therapeutic devices.
This vision is not without its challenges. However, the commitment from companies like EndoUVtech and CeramOptec, coupled with regulatory bodies’ increasing willingness to embrace well-substantiated innovations, creates a climate ripe for transformation. It is an invitation for all stakeholders—from clinicians to industrial engineers—to get into the conversation and actively participate in shaping the future of healthcare.
Conclusion: A Journey Toward Safer, More Effective Stroke Care
The journey toward effective and safe stroke treatment is long and often filled with overwhelming technical twists and turns. Yet, the developments we’ve seen from EndoUVtech and CeramOptec offer a beacon of hope for millions of patients worldwide who face the daunting aftermath of a stroke. By harnessing the power of UV laser technology and state-of-the-art optical fiber manufacturing, this collaboration is poised to bridge current treatment gaps in a manner that is both innovative and fundamentally patient-centered.
While the road ahead remains on edge with technical challenges, regulatory hurdles, and the need for extensive real-world data, this collaboration teaches us that every stride forward in medical technology is achieved by confronting the complicated pieces head-on. With continued research, open dialogue, and interdisciplinary support, the promise of UV laser stroke therapy could soon transform a nerve-racking challenge into a standard, effective approach to mitigate the long-term impacts of stroke.
As we continue to watch and report on these developments, one thing is clear: The future of stroke treatment is being rewritten by innovators committed to making high-precision, lifesaving therapies a reality. Through strategic partnerships, refined technological solutions, and a shared goal of improving patient outcomes, the effort to combat stroke and its devastating effects is now entering a bold new chapter—one where hope, science, and determination converge in a truly transformative way.
Originally Post From https://www.accessnewswire.com/newsroom/en/healthcare-and-pharmaceutical/endouvtech-announces-strategic-partnership-with-ceramoptec-to-advance-1064757
Read more about this topic at
Innovations spur post-stroke recovery
New approaches to recovery after stroke – PMC