Innovative Horizons in Diabetic Retinopathy Treatment
The recent approval of a Phase 2a study by the UK MHRA for the novel drug candidate ANXV has ignited considerable discussion in the eye care community. This development, centered on diabetic retinopathy and retinal vein occlusion, offers fresh hope for patients who have long grappled with these difficult eye conditions. It also opens a door to new treatment options that may help steer through the tricky parts of diabetic eye disease management.
As someone who has closely observed the evolution of modern medicine, I find this step both timely and exciting. With the potential to offer a shorter treatment cycle, the study not only challenges traditional approaches but also promises to shed light on key aspects of safety, tolerability, and early efficacy signals. In this opinion editorial, I will take a closer look at various aspects of the study, the adaptive design it embraces, and what it could mean for both the medical community and patients.
New Treatment Options for Diabetic Retinopathy: Assessing the Impact
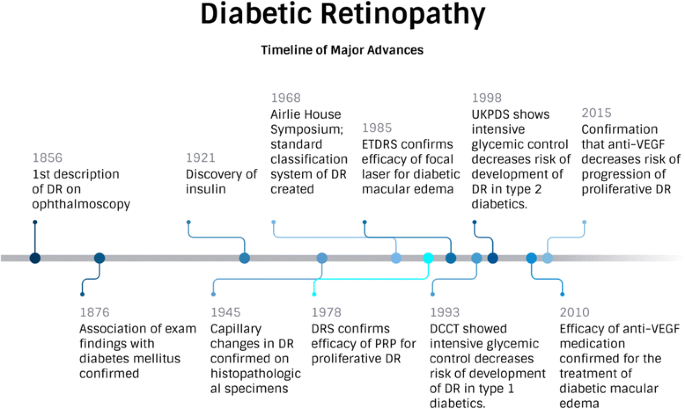
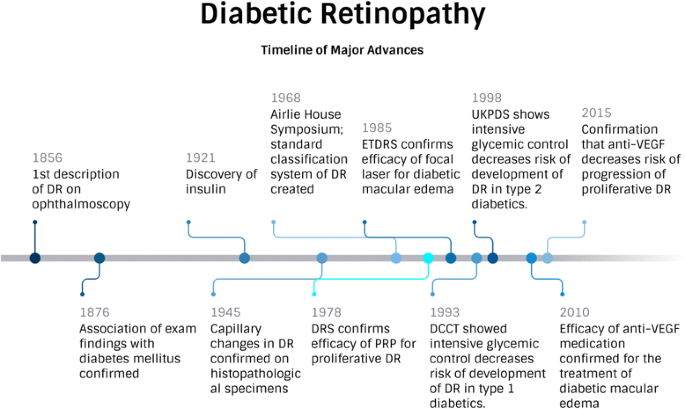
Diabetic retinopathy is a serious eye condition that arises when prolonged high blood sugar levels damage the small blood vessels of the retina. Such damage often leads to visual impairment and, in severe cases, blindness. The conventional treatment methods often face tricky parts, such as complicated pieces of prolonged treatment cycles and inconsistent patient outcomes. In this context, the introduction of ANXV in a controlled clinical study represents a beacon of promise.
Shorter Treatment Cycles: A Potential Game-Changer
One of the study’s central features is the focus on a shorter treatment cycle. Traditional treatment methods can be nerve-racking not just because of the lengthy and repetitive cycles but also due to the heavy dependence on frequent anti-VEGF injections. The new protocol, where patients receive ANXV over just 5 days with an immediate follow-up period, may address these challenges.
This shorter cycle has several potential advantages:
- Improved Patient Compliance: A concise treatment period can reduce the burden on patients and help them stick to their treatment plan more reliably.
- Early Assessment of Safety and Efficacy: By evaluating patients after just 30 days, doctors may quickly gather information on how well the treatment is working—a critical point when facing overwhelming treatment demands.
- Resource Optimization: A brisk treatment cycle can lead to cost-effective clinical evaluations, allowing more efficient use of both time and medical resources.
The possibility of a shorter treatment cycle is super important because it addresses some of the tangled issues that arise when long-term, repetitive regimens are hard for patients to manage. The approach may also dig into the fine points of patient comfort and overall treatment accessibility.
Adaptive Study Design: Pioneering a Flexible Clinical Approach
The adaptive clinical trial design employed in the ANXV study represents another innovative feature worth discussing. By allowing the evaluation of two indications—diabetic retinopathy and retinal vein occlusion—at the same time, this design offers a streamlined approach that can steer through several of the complex and confusing bits endemic to conventional study protocols.
This design provides several advantages:
- Time Efficiency: With parallel evaluation of two conditions, researchers can quickly factor in any early performance signals and adjust the study parameters accordingly.
- Cost-Effectiveness: When two indications are studied concurrently in a single clinic, resources can be allocated more effectively, reducing redundant efforts.
- Robust Data Collection: The ability to gather data on safety, tolerability, and potential efficacy from diverse patient groups in one go could pave the way for more refined future studies.
Adaptive design is full of opportunities to riddle less the tension between trial efficiency and patient safety. It allows key decision points regarding the next phase of studies, such as the Phase 2b trial, to be made with a more robust set of data based on the short-term, focused outcomes from the current trial.
Examining Clinical Study Outcomes: The Search for Early Efficacy Signals
Clinical outcomes form the backbone of understanding any new treatment’s potential. In the context of the Phase 2a study for ANXV, the focus lies on the early identification of safety and efficacy signals. The study seeks to monitor these signals through standardized tests that include best corrected visual acuity (BCVA), evaluations for retinal swelling, objective functional tests, and analyses of blood flow and vascular changes.
This careful examination of early results is seen as a crucial stepping stone. Here are some specific aspects that researchers are keen to poke around:
- Visual Acuity Tests: Measuring BCVA allows for the assessment of immediate improvements in a patient’s ability to see post-treatment.
- Assessment of Retinal Damage: By examining the degree of retinal damage caused by diabetes, clinicians can appreciate whether the treatment can potentially reverse or stabilize the condition.
- Retinal Swelling Measurements: Since swelling is a common and nerve-wracking symptom, its reduction is a positive sign indicating that the treatment might be alleviating the complicated pieces of diabetic retinopathy.
Experts are particularly excited about monitoring these early parameters. The identification of even slight differences in these tests after just 30 days of follow up can provide the fine shades of information necessary to improve future treatments and refine subsequent clinical assessments.
Patient-Centered Outcomes: Weighing the Risks and Benefits
The patient experience remains at the heart of any clinical breakthrough. Diabetic retinopathy is a condition that affects individuals in multifaceted ways, not just through the impairment of sight but also the resulting impact on their quality of life. With current treatment options often imposing a heavy time and quality-of-life burden, the new trial offers careful consideration of these holistic outcomes.
Several key points stand out as crucial for patients:
- Reduced Treatment Burden: A 5-day treatment cycle followed by a streamlined follow-up could lighten the ongoing load on patients, reducing anxiety and the intimidating nature of prolonged therapy.
- Focus on Safety and Tolerability: Close monitoring during a 30-day intensive period and additional follow-up for 90 days ensures that any adverse effects are caught early and managed promptly.
- Quality of Life Improvements: Early data accumulation helps physicians deliver better, more personalized treatment plans that could significantly enhance everyday functioning and overall well-being.
It is imperative that future studies continue to weigh both clinical outcomes and quality-of-life improvements equally. This balanced approach is critical to truly understanding a treatment’s efficacy—not just in measured clinical parameters but also in the subtle parts of everyday living that make a difference for patients.
The Role of Partnerships and Future Collaborations
Another intriguing element is how this study is paving the way for broader industrial partnerships while setting the stage for a potential Phase 2b study. The company behind ANXV, Annexin Pharmaceuticals, has clearly signaled that the data emerging from these early stages could inform extensive discussions with prospective industrial partners.
This dual-purpose focus—enhancing treatment indications while also preparing the market for future investments—is a strategy that balances immediate scientific inquiry with long-term business viability. Here are some strategic benefits of such partnerships:
- Resource Sharing: Collaborative trials can spread the load of expensive research and provide access to broader patient populations.
- Enhanced Credibility: Partnerships with established industrial players can lend a greater degree of trust and authority to the new treatment option.
- Streamlined Regulatory Approvals: Working with seasoned partners may help smooth out some of the complicated pieces that often make the regulatory approval process intimidating.
While the immediate focus remains on accumulating data from this Phase 2a study, the long-term implications of forming robust industrial partnerships cannot be overstated. They are key to making sure that promising early clinical results translate into real-world improvements for patients worldwide.
Understanding the Short-Term and Long-Term Implications
From the short-term perspective, the 30-day immediate follow-up and subsequent 90-day period are designed to capture the most critical data points regarding ANXV’s safety and potential efficacy. In these first few months, subtle improvements in vision, reduced retinal swelling, and better overall retinal health could all serve as promising indicators.
Over the longer term, however, a successful Phase 2a trial will act as a foundation for more expansive Phase 2b studies. This progression is essential for several reasons:
- Validating Long-Term Safety: While short-term data is crucial, understanding the effects of ANXV over months and even years will provide a deeper insight into its role in managing diabetic retinopathy.
- Fine-Tuning Dosage and Administration: Extended studies can help optimize the treatment regimen, ensuring that the dosing is both effective and tolerable for a wide range of patients.
- Broadening Treatment Indications: The dual focus on diabetic retinopathy and retinal vein occlusion in this trial may lead to expanded indications, benefiting even more patient demographics.
The layered approach—beginning with focused Phase 2a research followed by more comprehensive Phase 2b trials—ensures that each step is on firm ground. This methodical progression minimizes the risk of unexpected outcomes and allows researchers to figure a path forward by adapting strategies based on early signals.
Exploring the Underlying Mechanisms of ANXV in Retinal Disease
While the specifics of ANXV’s mode of action remain under ongoing investigation, early signals suggest that it targets the underlying vascular issues that cause diabetic retinopathy. By addressing the delicate blood vessels and promoting favorable blood flow, ANXV may help mitigate both the visual and physiological deteriorations associated with the disease.
In addition, the study aims to assess a number of key targets:
| Assessment Area | Description |
|---|---|
| Best Corrected Visual Acuity | Evaluates changes in vision following treatment. |
| Retinal Swelling | Determines reductions in swelling as a measure of treatment response. |
| Vascular Changes | Analyzes blood flow and vessel integrity which are crucial in diabetic retinopathy. |
By taking a closer look at these fine parts, the study hopes to provide insights into how ANXV may harmonize the retinal environment, leading to improved patient outcomes. Such detailed evaluations help to untangle the confusing bits that often arise when multiple indicators of treatment success are measured simultaneously.
Working Through the Challenges: The Patient and Clinician Perspective
For both patients and clinicians, any new treatment approach inevitably brings a mix of hope and caution. The nerve-racking nature of facing a chronic condition like diabetic retinopathy is compounded by the overwhelming task of sorting out the best treatment options. Nevertheless, a shorter treatment cycle combined with an adaptive study design has the potential to ease many of these tensions.
From the patient’s standpoint, the key benefits include:
- Fewer Hospital Visits: A 5-day treatment regimen significantly reduces the need for frequent hospital trips, which many patients find off-putting.
- Early Feedback: Regular and detailed follow-ups in the first month allow patients to see early changes, which can be incredibly comforting.
- Holistic Monitoring: The study’s design ensures that both visual performance and overall retinal health are closely tracked, giving patients a well-rounded picture of their progress.
For clinicians, the benefits extend to:
- Quick Decision-Making: The immediate data after 30 days can guide treatments, allowing for rapid adjustments if needed.
- Integrated Data Approaches: The use of standardized visual tests along with innovative imaging techniques helps in managing the fine details of each patient case.
- Streamlined Protocols: An adaptive study design minimizes the hassle of switching between different protocols for similar conditions.
Both perspectives emphasize the assessment of subtle trends in patient responses. This dual focus on quality of life and clinical efficacy is super important for long-term success and patient satisfaction.
Looking Ahead: Long-Term Innovation in Eye Care
This Phase 2a trial is not just a momentary scientific achievement—it is also a stepping stone towards broader innovations in the field of ophthalmology. If the early signals from the study prove positive, they could influence future research directions, funding decisions, and treatment regimens, ultimately contributing to improved standards of care in diabetic retinopathy and related conditions.
An overarching vision emerges from these developments:
- Greater collaboration between industry leaders and academic researchers is on the horizon.
- More adaptive clinical trial designs might become the norm, enabling faster and more efficient testing phases.
- Patients could soon benefit from treatments that not only manage their condition more effectively but also respect their time and overall well-being.
Innovations like ANXV remind us that the twists and turns in the path toward better health are best navigated with a spirit of experimentation and flexibility. Even when the road ahead seems loaded with issues, every small advancement gives us more confidence in our ability to find a path through the tangled issues of modern eye care.
Final Thoughts: Bridging the Gap Between Research and Real-World Application
In conclusion, the UK MHRA’s approval of the Phase 2a study for ANXV represents a meaningful leap forward in the management of diabetic retinopathy and retinal vein occlusion. With a focus on a shorter treatment cycle, personalized patient follow-ups, and an adaptive design that allows simultaneous evaluation of multiple indications, this study is set to provide meaningful insights into an area that has long been challenging.
It is crucial for both the medical community and patients to keep a watchful eye on the results of such studies, as they harbor the promise of turning overwhelming, off-putting treatment paradigms into more manageable, hopeful scenarios. The trial highlights the power of innovative thinking in overcoming the nerve-racking and tangled issues that patients face every day.
As we move forward, I encourage clinicians, researchers, and healthcare industry stakeholders to embrace the opportunities offered by adaptive trial designs and patient-centered approaches. The gradual accumulation of data—from early efficacy signals to long-term safety assessments—will be key in piecing together a treatment strategy that is not only scientifically sound but also profoundly humane.
Ultimately, the clinical journey from Phase 2a to potentially broader Phase 2b trials serves as a testament to the importance of partnerships, continuous data analysis, and above all, a commitment to improving patient lives. By finding your way through these challenging bits, we can cultivate advancements that not only restore vision but also restore hope.
Key Takeaways and Future Directions
In summary, here are the essential points to consider as we reflect on the implications of the ANXV Phase 2a study:
- Innovative Treatment Cycle: A short, 5-day treatment plan promises better patient compliance and earlier indication of treatment benefits.
- Adaptive Study Design: The ability to study diabetic retinopathy and retinal vein occlusion within a single trial exemplifies resourceful and efficient research methods.
- Patient-Centric Evaluation: Close follow-ups and detailed assessments point toward an improved understanding of quality-of-life improvements alongside visual acuity gains.
- Future Research Collaborations: Early results from this trial could attract key industrial partnerships, providing the necessary push for more extensive Phase 2b studies.
As research continues, it is essential to keep an open mind and a pragmatic approach to the fine points of clinical research. Each small piece of data can inform the next—making the cumulative progress in eye care both a science and an art.
Embracing a Future of Improved Eye Health
Looking forward, the narrative surrounding diabetic retinopathy is evolving quickly. With emerging therapies like ANXV, clinicians have an opportunity to rethink the conventional methods for a disease that has been traditionally tricky, loaded with challenges, and sometimes even overwhelming for patients. Innovations like these inspire us to dream of a time when managing diabetic eye conditions becomes simpler, less intimidating, and far more effective.
For healthcare professionals, the trial offers a chance to delve into the subtle details of patient responses and fine-tune treatments to individual needs. For patients, it provides a glimpse of hope—a prospect that science is imminently working to reduce the nerve-racking uncertainties associated with diabetic retinopathy. And for the research community, it reaffirms that adaptive, flexible study designs can indeed yield actionable, patient-centered insights.
Closing Reflections
In these evolving times, as we poke around the promising early findings of studies like this one, it becomes clear that the future of eye care lies in our ability to combine scientific rigor with empathetic, patient-first strategies. The collaboration among researchers, clinicians, and industrial partners is essential in steering through the intricate maze of diabetic retinopathy treatment options.
While it is still early days for ANXV, the adaptive and patient-focused approach of this trial lays a solid groundwork for future breakthroughs. As these early outcomes are scrutinized, refined, and eventually built upon, they may serve as the catalyst for major shifts in how we manage not only diabetic retinopathy but a range of other ocular diseases as well.
The journey from the clinical trial phase to mainstream treatment involves many steps—and each of those steps, no matter how nerve-wracking, is an essential piece of the broader mission to improve patient lives. With each new study and subsequent advancement, the gap between research and real-world application narrows, promising a brighter, clearer future for those affected by diabetic retinopathy.
As we continue to sort out these evolving treatment modalities, the message is clear: innovation, adaptability, and a patient-centered approach will be the pillars upon which the future of eye health is built. Let us celebrate these advancements, stay informed, and support the ongoing efforts to make effective, safe, and accessible treatments a reality for everyone affected by these challenging eye conditions.
Originally Post From https://www.ophthalmologytimes.com/view/uk-mhra-approves-phase-2a-study-for-anxv-for-diabetic-retinopathy
Read more about this topic at
Retinal Gene Therapy Options Advance Through Clinical …
Advancing Clinical Trials for Inherited Retinal Diseases