Balancing Digital Consent and Medical Innovations
In today’s fast-evolving digital landscape, transparency and user control have become super important when it comes to the way websites gather and process data. Equally, modern medicine is experiencing rapid advancements that offer new hope for patients while also posing tricky parts in regulation, manufacturing, and delivery of high-end therapies. These two seemingly separate worlds – the realm of digital consent and the cutting-edge biopharmaceutical sector – are converging in unexpected ways. In this opinion editorial, we will take a closer look at issues related to digital consent, including cookies and data processing, and then explore the growth in the Vascular Endothelial Growth Factor (VEGF) market, which is driven by rising cancer research and new therapies. Both of these topics underscore key themes: transparency, responsibility, and innovation in our modern age.
Understanding Digital Consent in a Data-Driven Age
Every time we visit a website, we are often greeted by messages asking us to accept cookies and other forms of data processing. This practice is not just a legal formality; it reflects a more profound shift in how digital content providers, advertisers, and even regulatory bodies manage and share data. As we figure a path through the tangled issues of digital consent, we must appreciate that such systems serve several important roles.
Why Cookies Matter for Data Transparency
Cookies help integrate content, external services, and third-party elements that enrich our online experience. In the background, this data collection feeds statistical analysis, personalized advertising, and even social media integration. At times, this data is shared with companies far beyond our national borders, including entities in other countries where privacy policies may be loaded with problems and hidden complexities. Such practices can be overwhelming or even nerve-racking for users who are not familiar with all the details.
The steps to opt in or out are usually straightforward: a simple click on “Accept All” or “Reject All” can control which cookies are enabled. However, many users may miss the fine points of how this data is filtered and shared. The process might seem intimidating, but it is a critical element in building trust between online services and their audience.
Clarifying the Consent Process
Most websites offer detailed options so that users can dig into the fine points of data processing. For example, along with the basic accept or reject options, you are often presented with advanced settings where you can pick and choose which aspects of data processing you are comfortable with. This level of choice is key for those who appreciate having the reigns in their own hands. Here are a few bullet points summarizing how cookie consent typically works:
- Essential Cookies: These are necessary for the website to function properly.
- Personalized Cookies: Help tailor the online experience by remembering your preferences.
- Advertising Cookies: Allow advertisers to create profiles and target ads more effectively.
- Analytics Cookies: Collect data to understand how users interact with the site.
This modular approach to data privacy ensures that users are not overwhelmed with a one-size-fits-all method. Instead, they can find their way through the layers of options to build a consent experience that reflects their own comfort levels.
Challenges and Responsibilities in Data Collection
While digital consent is often portrayed as a win-win for both users and service providers, there are still several tricky parts that need careful consideration. Companies collecting data must ensure that they clearly communicate how the data will be used. There is, however, a real risk that sensitive information might be accessible to authorities or organizations in countries with different privacy laws. Some users may consider this an off-putting prospect, as it introduces nerve-racking uncertainties regarding data security and personal rights.
Websites should therefore adopt robust privacy policies and transparency reports that address these concerns head on. The solution lies in maintaining an open dialogue with users, explaining not only what data is collected and processed but also how it benefits them by improving service delivery or providing personalized experiences. Through proactive communication, firms can demystify these tangled issues and earn the trust of their audiences.
The VEGF Market: A Midway Between Science, Innovation, and Commercial Success
Switching gears from digital data to modern medicine, one of the hottest topics in the biopharmaceutical arena today is the growing market for Vascular Endothelial Growth Factor (VEGF). This biomolecule plays a crucial role in angiogenesis – the process of blood vessel formation – making it super important for treating a range of conditions such as cancer, cardiovascular disease, and diabetes.
What is VEGF and Why Does It Matter?
VEGF is a protein that essentially helps promote the growth of new blood vessels. In healthy mechanisms, angiogenesis is critical for tissue repair and regeneration. However, in diseases such as cancer, VEGF can encourage the formation of blood vessels that feed tumors, contributing to their growth. On the flip side, in cardiovascular diseases, leveraging VEGF’s functions can help restore blood flow where it’s needed most.
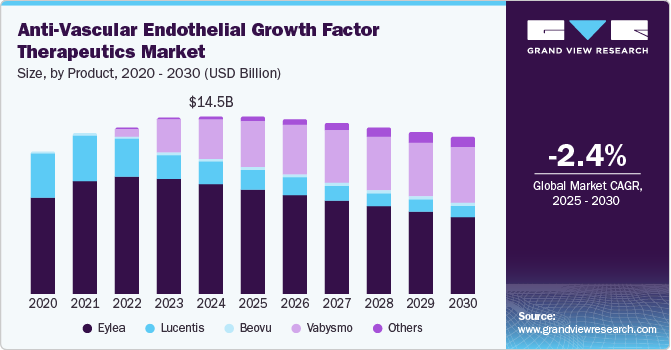
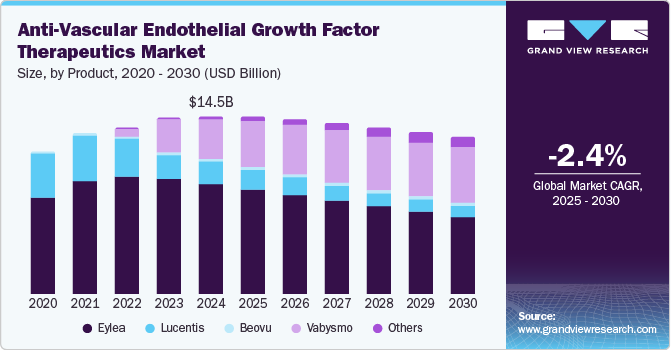
Recent advancements have seen VEGF become a focal point in research not only for its therapeutic applications but also for its potential in innovative drug delivery systems. With an estimated compound annual growth rate (CAGR) of 10.7% from 2022 to 2032, the VEGF market is rapidly expanding. Experts predict that by 2032, the market could reach a valuation of approximately US$590.6 million, up from US$213.6 million in 2022. This meteoric rise reflects both the promising scientific advances and the robust commercial interest behind VEGF-based therapies.
Expanding Horizons: Market Trends and Drivers
The growth trajectory of the VEGF market is driven by several interrelated factors. Rising incidences of vascular diseases, increased focus on regenerative medicine, and cutting-edge innovations in the field have all contributed to its increasing relevance. It is useful to lay out the key market drivers as follows:
- Advancements in Regenerative Therapies: New research into gene therapies, including VEGF-A mRNA treatments, is showing promise for conditions like ischemic heart disease and diabetes.
- Availability of Recombinant Proteins: The growing demand for recombinant proteins has paved the way for improved manufacturing techniques that help overcome the short half-life of VEGF.
- Increased R&D Investments: Significant investments by pharmaceutical companies and research institutes are fueling clinical trials that explore VEGF’s potential in treating various chronic conditions.
- Emerging Applications in Targeted and Personalized Medicine: As medicine becomes more tailored to individual patient needs, targeted therapies involving VEGF are emerging as key treatment options.
It is essential to recognize that while these trends signal strong growth, there remain a number of tricky parts. For instance, the short half-life of VEGF, along with the high production costs associated with its manufacturing, still presents some big challenges. However, innovative drug delivery systems – such as nanoparticles, liposomes, and gene delivery vehicles – are coming to the rescue, helping to mitigate these concerns.
Key Segments Within the VEGF Market
The VEGF marketplace is segmented based on product types and end-users. Human VEGF holds a dominant share, making up more than 60% of the market. This is not surprising given its critical roles in both physiological and pathological angiogenesis. Let’s break down the main segments:
| Category | Description |
|---|---|
| Human VEGF | Primarily used in both research and therapeutic applications, notably in oncology and regenerative medicine. |
| Mouse VEGF | Used in specific preclinical research models and niche clinical contexts, albeit with a smaller market share. |
| Other Types | Includes synthetic or modified forms that cater to specialized research and emerging therapies. |
On the end-user front, the market caters to a diverse group including pharmaceutical and biotechnology companies, research institutes, and contract research organizations (CROs). Pharmaceutical companies, predominantly based in North America and Europe, are at the forefront by leveraging VEGF in drug formulations, especially for cancer and cardiovascular diseases. Research institutes also form a significant portion of the market, given the expanding applications of VEGF in regenerative studies.
Regional Dynamics: North America and Beyond
The regional development of the VEGF market illustrates another layer of complexity. North America, and in particular the United States, remains the leader largely due to its robust R&D infrastructure and sizable investments in healthcare. Over 94% of the market in North America was attributed to the U.S. in 2021, a trend that is likely to continue given the high level of governmental and private support.
In Europe, Germany stands out as a key country driving innovation, especially with its strategic initiatives in recombinant protein production. These advancements have helped Germany secure a significant market share, contributing almost 28.8% of the European regional market in 2021. Other emerging markets, especially in Asia Pacific and Latin America, show promising potential as increased healthcare spending and wider access to medical services pave the way for new opportunities in VEGF-based therapies.
Challenges and Opportunities Within the VEGF Field
As with any burgeoning market, the VEGF field is loaded with both promising opportunities and its own set of challenges. Understanding these issues in a balanced way is key to unlocking the potential of VEGF therapies and ensuring that their benefits are realized for patients worldwide.
Short Half-Life Is a Tricky Part
One of the most confounding challenges in the application of VEGF-based therapies is its short half-life. The protein’s instability can significantly reduce its effectiveness, especially when administered intravenously or through local application. However, the current wave of research is focused on developing novel drug delivery systems, such as nanocarriers and lipid nanoparticle formulations, that can extend the effective period of VEGF in the body.
Efforts in this area are not just academic exercises but real-world solutions to a problem that has long held back the full potential of VEGF therapies. The development of these advanced delivery systems could ensure that VEGF reaches its target sites in a more sustained and targeted manner, ultimately enhancing therapeutic outcomes.
High Manufacturing Costs and Scalability Concerns
An additional hurdle facing the VEGF market is the high cost associated with manufacturing. Producing recombinant VEGF involves complicated pieces of biotechnological processes that require specialized equipment and highly controlled environments. This means that while the therapeutic benefits of VEGF are promising, scaling up production for widespread use – particularly in emerging markets – remains an intimidating challenge.
Companies are actively researching methods to optimize production, seeking to bring down costs without sacrificing quality. Innovations in bioprocess engineering and streamlined manufacturing protocols are expected to help address these tangled issues, making VEGF therapies more accessible and economically viable on a global scale.
Opportunities in Targeted and Personalized Medicine
The growing demand for targeted therapies is opening up new avenues for VEGF-based treatments. As medicine shifts more towards personalized strategies, the ability to tweak and modify VEGF formulations to meet specific patient needs represents a promising direction. Personalized therapies can potentially enhance treatment outcomes across various conditions such as cancer, cardiovascular diseases, and even some chronic kidney ailments.
Moreover, advancements in gene and cell therapy have created additional ways to integrate VEGF into broader treatment regimens. Researchers are actively exploring methods of VEGF gene delivery, which may revolutionize the way we think about regenerative medicine and targeted treatments. These novel approaches not only highlight the versatility of VEGF but also underscore the importance of continued research investment in this area.
A Glimpse Into the Future: Emerging Markets and Investment Trends
For the future, emerging markets in Asia Pacific and Latin America are expected to become key players in the VEGF landscape. Increased healthcare spending, improved research facilities, and a broader awareness about vascular health are likely to drive demand in these regions. Companies that can adapt to the local conditions while maintaining high standards of quality will be super important in capitalizing on these opportunities.
Investors are also taking notice. With the VEGF market showing a promising growth rate and offering lucrative opportunities, investment in biotechnological research and development related to VEGF is on the rise. Companies that can successfully get around regulatory challenges and streamline production processes are well-positioned to benefit from this growth.
The Synergy of Digital Transparency and Medical Innovation
While one may initially think that cookie consent on websites and the VEGF market have little in common, a deeper look reveals that both sectors are driven by similar underlying themes: the need for transparency, the demand for innovation, and the importance of user or patient empowerment.
Building Trust Through Transparency
In the digital age, where every click is monitored and data is continuously shared, websites that provide clear, understandable information about their data practices build a foundation of trust with their users. This openness is mirrored in the medical field, where transparency about clinical trial processes, potential side effects, and the limitations of therapies can significantly influence public trust in new treatments.
Both domains require a commitment to disclosing the nitty-gritty details – from how personal information is processed, to the small distinctions in treatment protocols. Users and patients alike deserve clarity when dealing with data or when considering their treatment options. The success of future innovations in both arenas hinges on this level of openness and accountability.
Leveraging Technology for Better Outcomes
The benefits of digital technology extend far beyond user data collection; similar advancements are also revolutionizing the field of medicine. In the case of VEGF therapies, cutting-edge technology has enabled the development of precise drug formulations and innovative delivery systems that overcome historical obstacles like the protein’s short half-life. In the digital world, innovative cookie management tools empower users to take control of their personal data.
Such technological innovations not only improve efficiency but also pave the way for more intimate, personalized experiences for users and patients alike. Whether it is by using advanced algorithms to target advertisements or designing new methods of delivering life-saving drugs, technology is taking center stage in both sectors.
Future Prospects: A Vision for a More Accountable and Innovative Tomorrow
Looking forward, the continued evolution of both digital consent practices and biopharmaceutical innovation will likely have a significant societal impact. On one side, continued improvements in cookie consent models promise a future where users become more empowered with the ability to manage their personal data. On the other, breakthroughs in VEGF research and allied areas promise revolutionary therapies that could transform the healthcare landscape.
Efforts in both areas highlight the importance of responsible management – whether it means responsibly handling personal data or responsibly developing potent therapies that address critical medical needs. As we move into a future where technology and healthcare increasingly intersect, it will be crucial for regulators, companies, researchers, and end-users to work together, ensuring that innovation does not come at the expense of transparency or safety.
Key Observations and Actionable Insights
After taking a closer look at the intersections of digital consent and medical innovation, several key observations emerge that are useful for both industry insiders and everyday consumers. These insights highlight best practices as well as cautionary advice on navigating these complex fields.
Recommendations for Digital Platforms
For websites and digital platforms, leveraging clear data management practices can go a long way toward building trust in an age when privacy concerns are high. Here are some actionable points:
- Clear Language: Use common and transparent language to explain data practices rather than relying on complicated legal jargon.
- User Empowerment: Provide granular control options that allow users to pick and choose what data they want to share.
- Regular Updates: Keep privacy policies updated, reflecting any changes that occur due to new technologies or regulations.
- Transparency Reports: Publish reports that detail how collected data is used, ensuring that no hidden complexities remain unaddressed.
In doing so, digital platforms not only protect themselves legally but also foster a relationship of trust and openness with their users. Transparent data practices are essential in a continually shifting digital landscape, setting a benchmark for accountability and ethical conduct.
Strategies for Advancing VEGF-Based Therapies
On the medical front, capitalizing on the potential of VEGF requires not only scientific innovation but also a strategic approach to address its tricky parts. Here are some strategies to consider:
- Invest in R&D: Continued investment in research is key for developing innovative drug delivery systems that can overcome VEGF’s short half-life.
- Collaborative Efforts: Encourage partnerships between biotech firms, academic institutions, and government agencies to share the load of high production costs and complex manufacturing processes.
- Targeted Approaches: Develop and fine-tune personalized therapies that precisely address specific conditions such as cancers or cardiovascular diseases.
- Expand Market Reach: Focus on emerging markets by adapting products to local needs and regulations, thereby broadening the available opportunities.
These strategies, coupled with ongoing technological and process innovations, could well set the stage for a new era of medical breakthroughs. Researchers and investors alike should remain open to adjusting their approaches as more is learned about both the benefits and the limits of VEGF-based therapies.
Integrating User-Centric Values in Innovation
Whether it is the digital space or the biotech sector, the ultimate goal is to ensure that innovation translates into tangible benefits for users and patients. In both contexts, the focus must always be on delivering a personalized, user-centric experience. In the digital world, this involves much more than simply collecting data – it is about genuinely empowering users with choices. In medicine, it means designing therapies that address patient-specific needs and ultimately improve quality of life.
This approach calls for a shift in perspective from mere process optimization to deeper human-centric values. Whether it is by giving users the ability to make informed decisions about their data or by tailoring therapies to suit everyday patient challenges, putting people first is the only way to ensure development that is both responsible and effective.
Final Thoughts: A Future Shaped by Trust and Innovation
As we face the coming years, the twin challenges of maintaining digital consent transparency and harnessing the potential of innovative therapies like VEGF remind us of the central role that trust plays in both realms. When users know that their data is managed carefully and when patients are offered therapies that are safe, effective, and tailored to their needs, the benefits extend not only to individuals but also to society at large.
The journey ahead may be filled with twists and turns, confusing bits, and off-putting challenges, but it is also rich with promise. As we continue to figure a path through tricky parts and make your way through complicated pieces of both data management and therapeutic innovation, we can look forward to a future where trust, accountability, and genuine progress remain at the forefront.
In Summary: Trust, Technology, and the Road Ahead
To recap, the digital consent model and the VEGF market represent two sides of the same coin: each is a field where transparency, innovation, and personalized approaches are key. The ability for websites to offer detailed cookie settings mirrors the medical industry’s need to offer detailed information and tailored treatments to patients. Both fields, however, face significant hurdles – from dealing with nerve-racking data privacy issues to managing tricky manufacturing costs and short product stability spans.
Below is a summary table that captures some of the core themes discussed:
| Area | Key Challenges | Potential Solutions |
|---|---|---|
| Digital Consent |
|
|
| VEGF Market |
|
|
This table serves as a quick reference to remind us that whether dealing with digital data or life-saving therapies, clear pathways and proactive choices are must-have elements for success. The decision-makers in both industries need to sort out these challenges by incorporating user-focused design and transparent practices.
The Importance of a Balanced Perspective
In closing, the convergence of digital consent and medical innovation is underpinned by a key idea: that progress is best achieved when ethical considerations walk hand in hand with technological breakthroughs. By addressing the tangled issues of cookie consent with clear, accessible language and by tackling the problematic pieces of VEGF therapy development head on, industry leaders can ensure that they not only keep pace with innovation but also earn the trust of their communities.
Whether you are a tech enthusiast concerned with online privacy or a healthcare professional involved in pioneering new therapies, the message remains clear: rigor, transparency, and the willingness to confront challenging bits head-on pave the way for a better, more trustworthy future.
Conclusion: Pioneering a Future Grounded in Transparency and Innovation
The developments in both the digital and medical arenas urge us to get into a dialogue about responsibility and progress. Embracing clear, user-focused approaches in collecting and processing data builds the kind of trust that is imperative in today’s rapidly changing world.
Simultaneously, the rapid growth seen in the VEGF market offers unparalleled hope for improved treatments for vascular diseases and cancers. With ongoing investments in research and technology, hurdles like a short half-life or intimidating manufacturing costs are being met with innovative solutions like advanced nanoparticles and gene therapies.
In essence, whether it’s ensuring that your online behavior is respected or that groundbreaking treatments are made accessible, the future is being shaped by those who understand the power of transparent, accountable practices. For digital platforms, this means enabling users to steer through their privacy settings confidently. For the biomedical community, it means adopting strategies that combine cutting-edge science with ethical responsibility. Both journeys are filled with twists and turns, but each step forward contributes to a more secure and innovative future.
As we continue to figure a path through the fine points of data management and medical research, we must stay committed to fostering environments where both technology and healthcare work in harmony for the betterment of society. The long-term promise is clear: a world where innovation does not intimidate, and where transparency and accountability are not mere buzzwords but integral aspects of our everyday lives.
Ultimately, the rise of the VEGF market alongside evolving digital data practices exemplifies how modern challenges can be overcome with ingenuity, persistence, and a steadfast commitment to ethical practices. Now more than ever, it is critical that all stakeholders – from tech developers to medical researchers – continue to put people first, ensuring that every advancement we make lights the way to a brighter, more inclusive future.
Originally Post From https://www.openpr.com/news/4146917/vascular-endothelial-growth-factor-vegf-market-driven
Read more about this topic at
Vascular Innovation Spotlight: Pedal Acceleration Time (PAT)
The Future of Vascular Care Innovations and Emerging …