Introduction to Abdominal Aortic Aneurysm and Vascular Health
The health of our blood vessels is a subject that remains both critical and, at times, a bit intimidating when we take a closer look at the research. Abdominal aortic aneurysm (AAA) is a serious condition marked by the abnormal dilation of the abdominal aorta, and although its progression can be nerve-racking, modern research is slowly unraveling its tricky parts. Recent investigations have shed light on the role that vascular smooth muscle cells (VSMCs) play in AAA, particularly through a process called pyroptosis—an inflammatory form of programmed cell death.
This opinion editorial will explore recent findings on how ALOX5, a key enzyme involved in lipid metabolism, influences VSMC pyroptosis and, in turn, impacts AAA formation. With insights into the NF-κB signaling pathway and its interaction with ALOX5, we can begin to piece together the tangled issues of inflammation and oxidative stress in AAA, offering hope for improved treatment strategies.
Understanding the Mechanism Behind VSMC Pyroptosis
The notion of cell death via pyroptosis can be overwhelming to those not familiar with the subject. Pyroptosis is an inflammatory form of programmed cell death distinguished from apoptosis by its association with severe inflammatory responses. Essentially, when VSMCs undergo pyroptosis, they release a cascade of pro-inflammatory factors that contribute to the damage of the aortic wall.
Recent studies explain that the excessive activation of inflammatory processes, triggered by stimuli such as angiotensin II (Ang II), leads to the formation of large pores in cell membranes. These pores eventually cause the rupture of the cell membrane and the release of intracellular molecules—further fuelling local inflammation. In the context of AAA, these confusing bits not only compromise the structural integrity of the aorta but also accelerate the process of aneurysm formation.
Key pro-inflammatory markers like IL-1β, IL-6, and IL-18, alongside robust oxidative stress markers such as reactive oxygen species (ROS) and malondialdehyde (MDA), often spike during this process. In a typical scenario of Ang II-induced injury, the increased expression of ALOX5 seems to serve as a catalyst for a vicious cycle of oxidative stress and inflammation, setting the stage for VSMC pyroptosis.
ALOX5: A Critical Enzyme in Lipid Metabolism and Inflammation
ALOX5 is one of the enzymes at the center of this research discussion. It catalyzes the peroxidation of polyunsaturated fatty acids like arachidonic acid—a process intimately linked to lipid oxidation. While this enzyme is ubiquitously found across various tissues, its upregulation in the aortic tissue of AAA patients points to a critical role in disease progression.
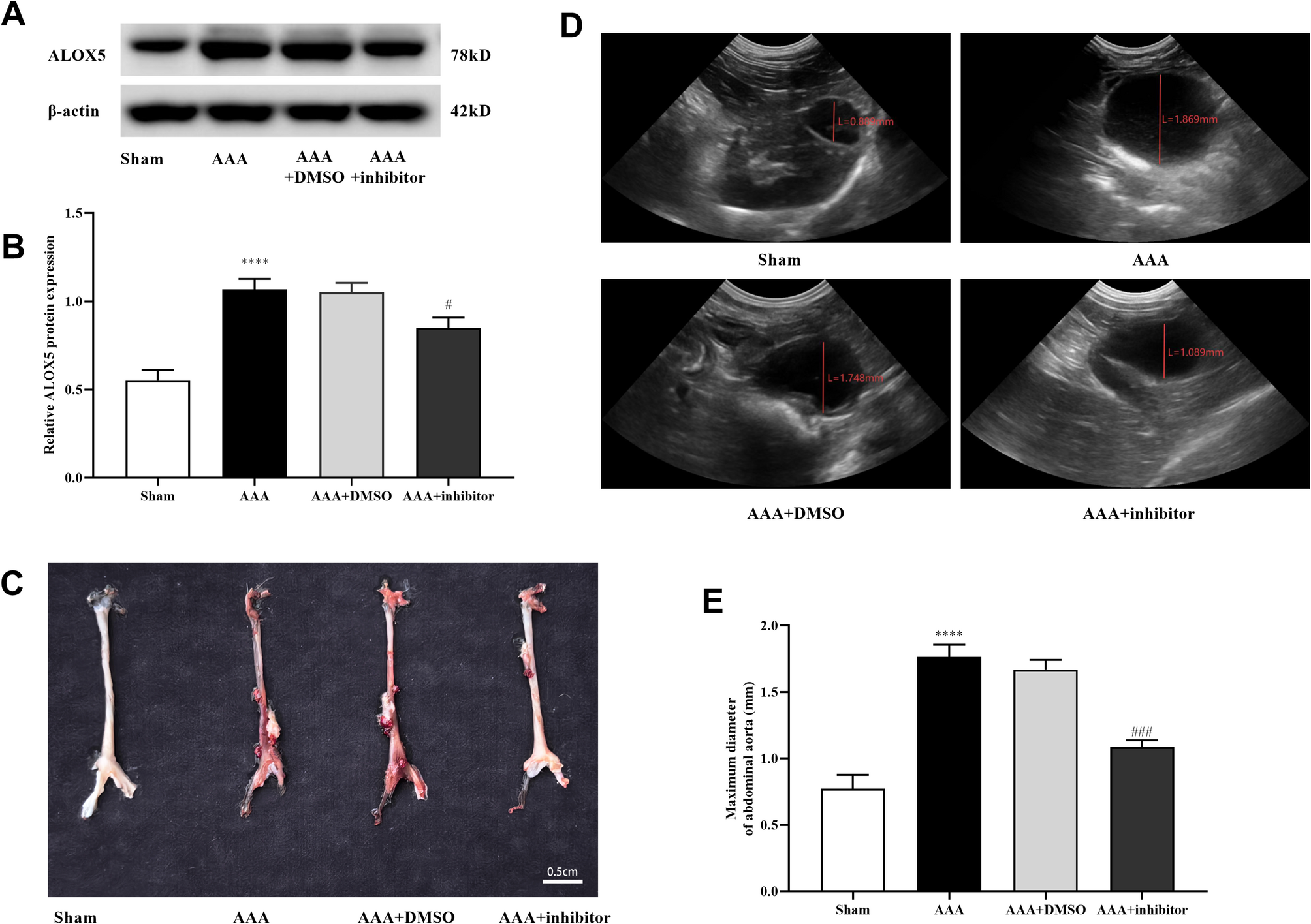
The observed increase in ALOX5 expression in AAA tissue suggests that it might be a key player in the inflammatory cascade. By augmenting oxidative stress and enhancing the release of pro-inflammatory cytokines, ALOX5 contributes to a state of chronic inflammation that creates a fertile ground for VSMC pyroptosis. Considering that AAA progression is heavily dependent on the cumulative effects of cell injury, inflammation, and oxidative damage, ALOX5 becomes a promising candidate for therapeutic intervention.
In laboratory studies, the inhibition of ALOX5 has been shown to reduce the diameter of the abdominal aorta in experimental models. This points to the possibility that targeting ALOX5 could limit the expansion of aneurysms and delay or even prevent their rupture. The enzyme’s central importance in both inflammatory signaling and oxidative stress pathways makes it an essential target when we consider innovative treatments for AAA.
Interplay Between ALOX5 and NF-κB: Finding a Path Through the Signaling Maze
Among the tangled issues related to AAA is the activation of the NF-κB signaling pathway. This pathway is renowned for its role in managing inflammatory responses and immune cell regulation. When activated, NF-κB triggers the transcription of genes that encode for various inflammatory mediators, thereby contributing to a state that is full of problems. In the case of AAA, the association between ALOX5 activity and the activation of NF-κB has emerged as a key area of interest.
Experimental studies indicate that the phosphorylated form of the NF-κB subunit p65 is markedly increased in diseased aortic tissues—a signal that the NF-κB pathway is active during aneurysm formation. When ALOX5 is inhibited, however, the activation of NF-κB is significantly reduced. This suggests that ALOX5 not only serves as a trigger for oxidative stress and inflammation but also operates upstream of NF-κB activation in VSMCs.
One can imagine NF-κB as a master switch that, once turned on, ignites various downstream pathways leading to pyroptosis. Inhibitors of the NF-κB pathway, such as BAY11-7082, have been demonstrated to reverse the harmful effects of ALOX5 overexpression, including the production of inflammatory cytokines and cellular oxidative damage. This relationship underlines the importance of looking not only at ALOX5 itself but also at the broader regulatory network in which it functions.
How ALOX5 Inhibition Could Transform AAA Management
From an opinion standpoint, the potential benefits of dampening ALOX5 activity in the management of AAA are quite promising. In animal models, the use of specific ALOX5 inhibitors has led to a visible improvement in the histopathological appearance of the aortic wall. Not only did inhibitor-treated subjects display a reduction in inflammatory cell infiltration, but they also showed less deposition of collagen fibers and fewer signs of oxidative stress.
In everyday terms, lowering ALOX5 activity appears to ease the overall harmful burden on the blood vessels. The table below summarizes some of the key effects observed with ALOX5 inhibition compared with untreated conditions:
| Parameter | Without ALOX5 Inhibition | With ALOX5 Inhibition |
|---|---|---|
| Abdominal Aorta Diameter | Increased | Reduced expansion |
| Inflammatory Cytokines | Elevated IL-1β, IL-6, IL-10, IL-18 | Significant decline |
| Oxidative Stress Markers | High ROS and MDA with low SOD activity | Lower ROS and MDA; improved SOD activity |
| Cell Pyroptosis Markers | Upregulated NLRP3, caspase-1, ASC | Downregulated expression |
This table underscores how intervening in one of the key processes—by blocking ALOX5—can have cascading beneficial effects that reduce the risk of aneurysm progression. By preventing the excessive activation of NF-κB and limiting the oxidative and inflammatory burden on the vessel wall, we could potentially steer through the dangerous twists and turns of AAA development.
Exploring Alternative Treatment Strategies: The Role of Inflammation and Oxidative Stress Reduction
Beyond targeting specific enzymes like ALOX5, a broader approach to mitigating AAA progression might include reducing inflammation and oxidative stress in general. There are several complementary strategies that clinicians might consider in the search for improved AAA management:
- Antioxidant Therapy: Interventions aimed at boosting antioxidant enzymes such as superoxide dismutase (SOD) could help neutralize reactive oxygen species before they cause severe damage.
- Anti-inflammatory Agents: Medications that temper the overproduction of inflammatory cytokines may alleviate the local inflammatory state that contributes to aneurysm progression.
- Lifestyle Modifications: Diet, exercise, and control of blood pressure are all key components for overall vascular health; these factors may indirectly reduce the risk associated with oxidative damage and inflammation.
- Gene Regulation and RNA-based Therapies: Emerging strategies in gene editing or RNA interference (such as the use of siRNA targeting ALOX5) show promise in fine-tuning the inflammatory pathways at the molecular level.
Each of these approaches can work in tandem with the inhibition of enzymes like ALOX5 to offer a more holistic management plan for patients at risk of AAA. While the current body of research focuses extensively on the molecular level—identifying markers and signaling pathways—the ultimate goal remains to translate these findings into practical, effective treatments that can be incorporated into everyday clinical practice.
Diving Into the Tricky Parts: Challenges in Targeting Molecular Pathways
As promising as it sounds, targeting molecular mechanisms such as ALOX5 activity and NF-κB signaling is not without its tricky parts. One challenge lies in the fine points of the pathway interactions; the roles of oxidative stress and inflammation in AAA are loaded with issues that make a straightforward intervention difficult. Researchers face several tangled issues including:
- Off-target Effects: Inhibitors may inadvertently affect other essential pathways, thereby leading to unforeseen side effects.
- Variability in Patient Responses: Individual genetic differences mean that what works for one patient might not work for another.
- Drug Delivery Challenges: Ensuring that sufficient concentrations of the inhibitor reach the target tissue safely is a significant challenge in experimental models and will be equally complex in human subjects.
- Long-term Safety: Chronic inhibition of pathways like NF-κB, which play a key role in immune response, might compromise the body’s ability to fend off infections or perform regular cell maintenance.
There is also the subtle fact that many of these interventions were tested in animal models like the Ang II-induced ApoE knockout mice, and the translation to human pathophysiology requires careful consideration. That being said, the accumulated evidence still points toward a promising role for agents that can suppress ALOX5 activity.
Sorting Out the Research: A Look at Experimental Evidence
Experimental research in the field has employed several techniques to establish the role of ALOX5 in AAA. Researchers have worked through the following key methods to break down the process:
- Histological Staining Techniques: Hematoxylin-eosin and Masson staining have been used extensively to document the disordered structure of the vascular wall, collagen deposition, and inflammatory cell infiltrates in the presence of AAA.
- Biochemical Assays: The measurement of oxidative stress markers such as ROS, MDA, and SOD activity offers insights into the biochemical environment of the disease state.
- Western Blotting and RT-qPCR: These methods help quantify the levels of key proteins such as ALOX5, NLRP3, caspase-1, and components of the NF-κB pathway, allowing researchers to gauge changes under different treatment conditions.
- Cell Culture Studies: By using cultured MA-VSMCs treated with Ang II, scientists have been able to directly observe how ALOX5 expression influences pyroptosis and inflammatory responses.
Collectively, these experimental findings present a cohesive picture: when ALOX5 is overexpressed under the influence of Ang II, detrimental changes occur that lead to VSMC death, increased inflammation, and ultimately, the expansion of the aorta. Conversely, silencing or inhibiting ALOX5 curtails these changes, underscoring the enzyme’s super important role in mediating the harmful effects seen in AAA.
Charting a Course Through the NF-κB Signaling Maze
As we get into the fine shades of the NF-κB signaling pathway, it becomes evident that this system is a driving force in the management of inflammatory responses. The activation of NF-κB in response to elevated levels of reactive oxygen species and inflammatory cytokines is a typical example of a feedback loop that can become tense if not properly regulated.
When the NF-κB pathway is activated, it travels to the cell nucleus to turn on the genes responsible for further inflammatory responses. This chain reaction can be especially detrimental in the delicate environment of the vascular wall. From a therapeutic perspective, the possibility of using NF-κB inhibitors to dampen this loop is an enticing strategy. Research has shown that when NF-κB activity is reduced—either directly via pharmacological inhibitors like BAY11-7082 or indirectly through the suppression of upstream regulators like ALOX5—the inflammatory cascade is significantly attenuated.
The table below offers a simple breakdown of the relationship between ALOX5, NF-κB, and VSMC pyroptosis:
| Component | Role in AAA | Effect of Inhibition |
|---|---|---|
| ALOX5 | Triggers lipid peroxidation and inflammation | Reduces oxidative stress and inflammatory cytokine release |
| NF-κB | Activates genes for inflammatory mediators | Lowered expression leads to reduced inflammation and pyroptosis |
| Pyroptosis Markers (NLRP3, caspase-1, ASC) | Signify VSMC death and release of inflammatory agents | Decreased expression helps maintain vascular integrity |
This organized summary highlights how managing one component in the pathway can have an array of beneficial effects on every downstream event. It reinforces the concept that a targeted approach—aimed at mediating early regulators like ALOX5—has the potential to significantly slow AAA progression.
Taking a Closer Look at the Future of AAA Research
Despite these exciting developments, the journey to a full understanding of AAA is not without its complicated pieces. There are several areas where researchers still need to poke around in greater detail:
- Mechanistic Diversity: While the connection between ALOX5 and NF-κB is compelling, other pathways and factors may also play hidden roles in triggering VSMC pyroptosis.
- Therapeutic Targeting: The translation from animal models to human application is loaded with issues. Safety profiles, drug interactions, and long-term effects remain areas that require further exploration.
- Personalized Medicine: Given the patient-to-patient variability, future research must consider how genetic and environmental factors come together to influence AAA progression.
- Combination Therapies: A multi-pronged approach that targets both inflammatory signaling and oxidative stress, possibly in combination with lifestyle changes, could be key to future treatment strategies.
Continuing to get into the little details is essential if we are to figure a path towards a comprehensive treatment strategy. A multidisciplinary approach, combining modern molecular techniques with clinical insights, offers the best chance for meaningful progress against this life-threatening disease.
Managing Your Way Through the Clinical Implications of the Research
From a practical standpoint, clinicians are faced with the nerve-racking challenge of treating a condition that, until recently, had few non-surgical options. The potential for pharmacological intervention with agents that inhibit ALOX5 activity opens up a new era in AAA management. Such treatments may be used as an add-on to the traditional surgical methods, ultimately reducing the incidence of aortic rupture and improving patient outcomes.
Here are some key considerations for translating these findings into clinical practice:
- Risk Stratification:
- Early identification of patients with elevated inflammatory markers and high ALOX5 expression is crucial.
- Advanced imaging techniques could be used to monitor changes in the aortic diameter over time.
- Integrated Treatment Plans:
- Combining pharmacotherapy with lifestyle interventions may yield better results.
- Regular monitoring of oxidative stress markers and inflammatory cytokines can aid in fine-tuning the treatment regimen.
- Personalized Medicine Approaches:
- Genetic screening might eventually help to predict which patients will benefit the most from ALOX5 targeting strategies.
- The development of tailored inhibitors that minimize off-target effects is a promising area of future research.
Such a well-rounded treatment approach represents a significant advancement over the current methods, which primarily revolve around surgery. By finding your way through the intricate pathways involved in AAA, there is hope for alternative treatments that are less invasive and more effective in the long run.
Opinions on the Current State of AAA Research
In my view, the current research into the mechanisms underlying AAA, particularly studies focusing on ALOX5 and VSMC pyroptosis, paints an encouraging picture. By tackling the problem at its source—addressing the inflammatory and oxidative triggers early on—there is real hope for reducing both the occurrence and severity of aneurysms.
There is a growing consensus among scientists that successful management of AAA will require a multi-targeted approach. With key players such as ALOX5 and NF-κB in the spotlight, the future of AAA research looks to be shifting from broad-spectrum anti-inflammatory strategies to more precise, molecularly guided interventions. Such precision is necessary to make sense of the many twisted parts in the disease process.
One of the most exciting aspects of these developments is the potential to move away from the purely surgical model of AAA treatment. Surgery, though often life-saving, is associated with high risks, intimidating complications, and long recovery periods. Robust pharmacological therapies stand to revolutionize the patient experience, offering less invasive and more sustained treatment benefits.
On the flip side, it remains critical to recognize that these studies are just one piece of the overall puzzle. With many of the findings coming from controlled laboratory settings and animal models, the road to human application is long and full of challenges—but not insurmountable ones. Ongoing clinical trials and further research will be essential to confirm the benefits observed in preclinical models and to translate them into tangible patient outcomes.
Digging Into the Broader Implications for Modern Medicine
The ramifications of this research extend far beyond treatment for AAA alone. The insights gained from studying the role of ALOX5 and the NF-κB pathway in vascular smooth muscle cell pyroptosis have broader applications in modern medicine. Chronic inflammation and oxidative stress are hallmarks of many cardiovascular diseases, and the lessons learned here could be extrapolated to conditions such as atherosclerosis, heart failure, and even certain neurodegenerative disorders.
With the growing prevalence of cardiovascular diseases worldwide, innovative approaches that target the root causes of these conditions are in high demand. By employing targeted therapies that reduce inflammation and oxidative stress, clinicians may be able to prevent the onset of a host of diseases, making these findings a potential game-changer in preventative medicine.
Moreover, by understanding how pathways like those controlled by ALOX5 and NF-κB interact, medical professionals can better design combination therapies that address multiple aspects of disease progression simultaneously. It is clear that the management of cardiovascular conditions requires a balanced approach that manages both the triggers and the responses to oxidative and inflammatory damage.
Future Directions and Research Challenges
While the current evidence is promising, several challenges remain. Future research needs to address the following questions and areas of uncertainty:
- Molecular Specificity: What are the fine details governing the interactions between ALOX5, NF-κB, and the inflammatory mediators? The subtle parts of these molecular interactions require detailed mapping.
- Long-term Effects: How safe is chronic ALOX5 inhibition in the context of long-term patient management? The balance between suppressing harmful inflammation and preserving normal cellular functions is delicate.
- Alternative Pathways: Are there other parallel signaling routes that need to be targeted concurrently? The landscape is full of confusing bits, and researchers must dig into other potential mechanisms that could be contributing to AAA progression.
- Human Trials: Translating preclinical findings into human clinical trials will be a major step. Large-scale, randomized controlled studies are needed to confirm the efficacy and safety of these interventions.
- Combination Therapies: How can we best combine pharmacological interventions with lifestyle modifications and surgical techniques to optimize patient outcomes? Future research will have to consider integrated treatment designs.
These questions are not meant to discourage further investigation but rather to highlight the nerve-racking yet necessary journey ahead. The steps involved in figuring a path through these complex mechanisms will ultimately lead us to treatments that can save lives and enhance the quality of healthcare.
Final Thoughts on Tackling Abdominal Aortic Aneurysm
In conclusion, the research pointing to ALOX5 as a regulator of vascular smooth muscle cell pyroptosis represents a significant advance in our understanding of AAA. By appreciating the key role of ALOX5 in mediating inflammatory responses through the activation of pathways like NF-κB, we can begin to untangle the myriad issues that have long rendered AAA a formidable challenge in vascular medicine.
While the twists and turns of this research are full of tricky parts and intimidating complexities, the potential rewards are equally substantial. It is clear that innovative treatments based on these findings could herald a new era in the management of AAA—one where the progression of the disease is halted before it reaches a point of no return, and where patients may have viable alternatives to high-risk surgical interventions.
As our understanding of the fine points of molecular signaling deepens, there is a strong argument to be made for using a multi-faceted approach that incorporates ALOX5 inhibition, NF-κB modulation, anti-inflammatory therapies, and lifestyle adjustments. Each of these components offers a piece of the solution, and together, they provide a comprehensive strategy against the nerve-racking progression of AAA.
Ultimately, embracing these advances in research requires a commitment to ongoing study and the translation of laboratory insights into clinical practice. Though the road ahead is certainly loaded with challenges, the progress made thus far gives cause for optimism. With careful experimental design, rigorous testing, and a collaborative approach between scientists and clinicians, the future of AAA treatment looks poised to take a significant leap forward.
Summary: Key Takeaways for Clinicians and Researchers
To summarize, here are the essential points that emerge from current research on ALOX5 and AAA:
- Mechanism Insight: ALOX5 promotes VSMC pyroptosis via oxidative stress and inflammatory cytokine release, largely through the activation of the NF-κB pathway.
- Therapeutic Potential: Both ALOX5 inhibitors and NF-κB blockers have shown promising results in reducing inflammatory markers, oxidative stress, and aneurysm size in experimental models.
- Translational Challenges: While animal models provide valuable insights, more research is needed to ensure these findings translate safely and effectively to human patients.
- Holistic Strategy: Future treatment of AAA will likely require a combination of targeted pharmacological therapies along with traditional surgical techniques and supportive lifestyle modifications.
- Future Research Needs: Improved understanding of the subtle details of this signaling cascade is important, including potential off-target effects and long-term safety profiles.
By taking a closer look at these interconnected aspects of AAA progression, the medical community can better steer through the challenges and move closer to effective, less invasive treatments for this life-threatening condition.
Conclusion: A New Direction in AAA Management
The research into ALOX5’s regulation of vascular smooth muscle cell pyroptosis offers a fresh take on a problem that has long been shrouded in intimidating complexities. With the proper application of this knowledge, clinicians are now better equipped to manage and potentially prevent the dangerous expansion of abdominal aortic aneurysms.
We are witnessing a time when molecular biology meets clinical practice, and the fine points of cellular signaling are starting to translate into real-world treatments. It is a journey filled with subtle parts, tangled issues, and nerve-racking challenges—but it is also one that holds the promise of safer, more effective therapeutic strategies that could one day save countless lives.
In this evolving landscape, the integration of targeted inhibitors, personalized medicine, and a deeper understanding of inflammatory pathways stands out as a beacon of hope. While many questions still remain, the current findings create a path forward that is as promising as it is necessary.
As we continue to dig into the research and piece together the overlapping narratives of inflammation, oxidative stress, and cell death, one thing remains clear: the fight against AAA is entering a new era. With each incremental discovery, the prospects of taming this formidable disease become brighter, offering a safer and healthier future for patients worldwide.
Originally Post From https://www.nature.com/articles/s41598-025-14268-6
Read more about this topic at
ALOX5 regulates vascular smooth muscle cells pyroptosis …
ALOX5 drives the pyroptosis of CD4 + T cells and tissue …