FDA Approval of Embrace Hydrogel Embolic System: A Game Changer for Hypervascular Tumor Treatment?
The recent U.S. FDA premarket approval for the Embrace Hydrogel Embolic System marks a significant evolution in interventional oncology. As we take a closer look at this breakthrough, we find ourselves with not only a newfound tool in the interventional radiologist’s kit but also an opportunity to question how emerging embolic technologies reshape the treatment of hypervascular tumors. In this opinion editorial, we dive in into the details, exploring the trial outcomes, clinical benefits, and potential pitfalls of the technology, along with some thoughts on how data privacy—down to cookie consent details on medical websites—plays a role in today’s digital healthcare landscape.
The approval process is never a simple ride; it comes with twists and turns, and the regulatory journey is often loaded with tricky parts. However, the Embrace system’s journey, backed by data from a prospective, randomized, multicenter pivotal study, offers us a clear case study of how industry innovation can benefit patients suffering from hypervascular tumors.
U.S. FDA Premarket Approval and the Future of Liquid Embolic Devices
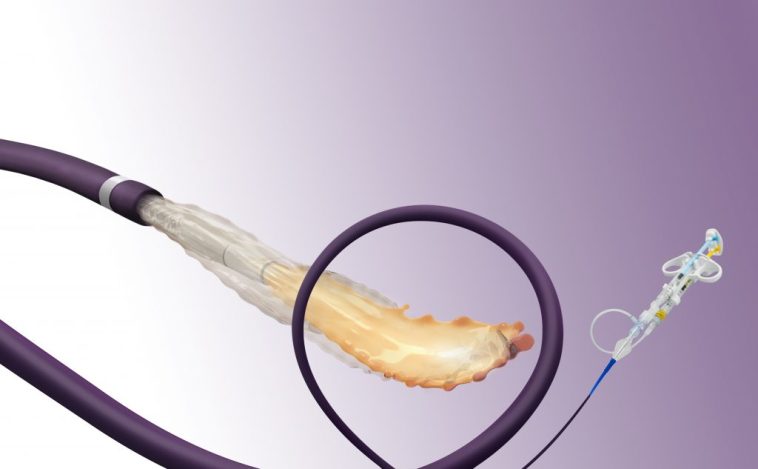
As the first liquid embolic device approved specifically for hypervascular tumors in peripheral arteries ≤5 mm, the Embrace Hydrogel Embolic System represents a breakthrough that many experts in interventional radiology have been eagerly anticipating. Instead of viewing these new materials as just another tool, the approval sparks a broader discussion around modern medical device innovations. The device utilizes two low-viscosity aqueous liquid precursors that polymerize in situ—forming a soft polyethylene glycol (PEG) hydrogel that ultimately blocks off blood flow to targeted tumors.
This regulatory milestone puts the spotlight on several important factors:
- Deep penetration into the tumor’s small vessels
- Avoidance of catheter entrapment
- Lack of imaging artifacts to allow clear follow-up diagnostics
- High technical success and safety profile as evidenced by a 99% freedom from major adverse events
Each of these aspects contributes to the increasing credibility of interventional oncology techniques that are now focused on more precision-targeted therapies. As clinicians begin to work through the troubleshooting and tangled issues that often accompany the introduction of new technology, the Embrace HES appears to simplify many of the traditionally nerve-racking challenges of embolization procedures.
Hypervascular Tumors: Understanding the Tricky Parts of Treatment
Hypervascular tumors, known for their abnormal blood vessel growth in organs like the liver, kidney, and bone, introduce a host of confusing bits when it comes to treatment. The high vascularity of such tumors makes surgical removal a daunting task due to the increased risk of bleeding. Typically, practitioners have turned to options like transcatheter arterial embolization (TAE) and transarterial chemoembolization (cTACE) for patients with progressive disease or those requiring localized tumor control. However, these methods sometimes come with their own set of complicated pieces—the unpredictable performance of traditional embolic agents and the possibility of non-target embolization.
When analyzing the impact of the Embrace HES system, it becomes clear that its unique design holds promise in mitigating many of the common risks associated with embolization. The soft hydrogel forms within the vessel, sealing off the tumor’s blood supply, and at the same time, aids in reducing the likelihood of adverse outcomes. This breakthrough is especially significant when one considers that even the smallest vessel diameters—down to 10 microns—can be addressed with the new system.
Despite its promise, this approach is not without its own set of challenges. As with any new treatment modality, there are a few tricky parts that require careful consideration:
- The learning curve associated with the simultaneous injection of two precursors
- Ensuring consistent polymerization within different vascular environments
- Monitoring and addressing any delayed reactions or potential device-related issues
These issues remind us that while innovation often brings essential improvements, they also come bundled with a handful of subtle details and hidden complexities that practitioners must figure a path through before full integration into standard care.
Advancements in Interventional Radiology: Deep Penetration and Precision Technology
One of the super important components of the Embrace HES system is its ability to deeply penetrate the tumor’s vascular bed. Traditional embolic agents often struggle when it comes to reaching the finest vessels, but Embrace HES is designed to address this precise challenge. By delivering two liquid precursors that form a cohesive hydrogel in situ, the technology offers a promising solution that minimizes the probability of non-target blockage—one of the nerve-racking pitfalls seen with older methods.
Interventional radiologists appreciate a device that not only acts effectively but also leaves minimal aftereffects in follow-up imaging. The absence of image artifacts allows physicians to clearly visualize the tumor and assess the embolization’s success. In addition, a high technical success rate (88.6% as confirmed by an independent core laboratory) coupled with a near-perfect safety margin further instills confidence among clinicians who might be hesitant to adopt new techniques due to intimidating trial outcomes.
Moreover, the design of this device signifies a subtle yet crucial shift towards personalized interventional oncology. Medical professionals are increasingly expected to find their way through complex treatment protocols, and innovations like Embrace HES provide them with a robust tool to do just that.
Clinical Trial Insights: Safety, Effectiveness, and the Road Ahead
The pivotal trial that led to the approval of Embrace HES involved 150 patients from 22 institutions around the globe. These patients, diagnosed with hypervascular tumors, were randomized in a 2:1 ratio to receive treatment with either the Embrace HES system or the standard of care (TAE or cTACE). Results from the study were promising, indicating both high effectiveness in technical success and a strong safety profile with 99% freedom from major adverse events.
It is important to point out that while no adverse event was classified as device-related alone, the intricate process of clinical evaluation is always full of little twists and turns. In this study, the dual benefits of deep penetration and cohesive embolization provided a fresh perspective on managing these life-threatening tumors. Physicians are encouraged to digest the fine points of the trial outcomes to better understand the value proposition that this device offers.
The implications of the study stretch beyond just the trial’s results. They signal a broader trend in the healthcare industry where new technologies must prove themselves under both standard testing protocols and in real-world settings marked by complicated pieces and subtle details. For many in the healthcare community, the Embrace HES system may serve as a case study in how innovative design and rigorous testing protocols can come together to create transformative treatment options for patients.
Interventional Oncology and the Promise of Liquid Embolic Therapy
There is little argument that interventional oncology is becoming a central pillar in modern cancer treatment. In a field that is constantly working through challenging issues, highly innovative devices like the Embrace Hydrogel Embolic System offer a fresh perspective. The system’s ability to minimize non-target embolization and reduce the risk of catheter entrapment is a critical advantage over older liquid embolic agents, addressing many of the confusing bits typically encountered during embolization procedures.
One of the key benefits that many experts tout is the system’s capacity to offer precise embolization. When facing the tricky parts of hypervascular tumor management, precision is more than just essential—it is the must-have element that determines overall treatment success. As the technology matures, the hope is that interventional radiologists will work through any remaining hurdles and successfully incorporate such advanced systems into routine practice.
Nonetheless, while the preliminary data are promising, there remains a need for ongoing post-market surveillance and additional clinical research. It is only by continuously monitoring outcomes that clinicians can get around the unpredictable issues sometimes encountered with new drug-device combinations or procedural techniques.
The Overlooked Role of Data Privacy in Healthcare Communications
While the excitement around new interventional devices like the Embrace HES system is palpable, there is another important aspect of modern healthcare communications that deserves attention: data privacy. Medical news websites and healthcare journals routinely implement cookie consent banners and detailed data handling policies to protect user information. Although these mechanics might seem off-putting and loaded with confusing bits, they are critical in building trust among readers.
For example, websites such as BioSpace present detailed information regarding cookie usage in clear, structured formats. Tables listing different cookies, their purposes, storage durations, and types help readers figure a path through the web of privacy settings. Some of these details include:
- Necessary cookies for maintaining basic functionalities like page navigation
- Preference and statistics cookies that track user behavior for improvement
- Marketing cookies used to tailor content and advertisements
In an era when data breaches and privacy concerns are more intimidating than ever, transparency in data usage and the provision of user-friendly settings have become super important. Healthcare organizations must ensure that the benefits of modern interventional techniques and clinical advancements are matched by equally robust digital protections. After all, the trust of healthcare professionals and patients alike is built on the assurance that personal data is handled with care and clarity.
Understanding the Value of Transparent Data Practices
Medical websites that are committed to transparency do more than simply notify users about cookie policies—they offer detailed insights into how user data is collected, stored, and managed. This approach is not only key in building trust but also critical for fostering informed engagement from the public. Some of the useful practices include:
- Clear categorization of cookies into necessary, preference, statistics, and marketing segments
- Tables outlining cookie names, providers, storage durations, and types
- Easy access to privacy policies and consent management tools
- Guidelines that help users change their consent preferences anytime
Understanding these fine points allows readers to truly grasp the little details that make up the digital experiences behind manipulating healthcare content. For a field as dynamic and sensitive as medicine, it is critical that both information providers and consumers figure a path to a transparent and secure online experience.
Taming the Challenges of New Medical Device Implementations
The journey from innovation to clinical implementation is always full of challenging issues. As hospitals and clinics begin to adopt the Embrace HES system, practitioners will need to work through a series of nerve-racking training sessions and integration processes. Some of the hurdles include:
- Training staff on the specifics of handling dual-component liquid precursors
- Ensuring that new protocols do not interfere with existing treatment pathways
- Adjusting imaging protocols to correctly assess the post-embolization status
- Coordinating multi-disciplinary teams to minimize potential issues arising during procedures
These tricky parts are typical of any new technology entering the complex landscape of modern medicine. While the benefits are promising, medical professionals must be prepared to invest time in getting familiar with every small twist and little distinction that differentiates the Embrace HES system from existing devices.
This process of learning and adaptation is essential for truly leveraging the advanced features of new embolic agents. By fully understanding the hidden details, clinicians can ensure that treatment protocols are optimized, enhancing patient outcomes while reducing the risk of complications associated with off-target embolization.
Comparing Embrace HES with Conventional Embolization Techniques
An essential part of forming an informed opinion comes from comparing new innovations with conventional methods. Traditional embolic systems like TAE or cTACE have been the standard for many years, yet they do present several challenges:
- Limited Vessel Penetration: Many conventional agents lack the ability to deeply penetrate the microvasculature of hypervascular tumors.
- Higher Risk of Non-Target Embolization: These systems sometimes inadvertently embolize non-target tissues, potentially leading to complications.
- Imaging Artifacts: Following treatment, residual artifacts can complicate follow-up evaluations, clouding the accurate assessment of tumor response.
On the other hand, the Embrace HES system demonstrates the potential to address many of these issues by:
- Offering deeper, more uniform penetration into the tumor’s blood network
- Minimizing the chance of unintended embolization, thanks to its in situ polymerization
- Eliminating imaging artifacts, which allows for a clearer post-procedural evaluation
This comparison highlights why many experts consider the Embrace HES system as both innovative and timely. The device’s design tackles the twisted issues that have long hampered the effectiveness and safety of earlier embolic agents, ushering in a new era of precision interventional radiology.
Addressing Concerns: Training and Adaptation in Clinical Settings
No new technology is without its learning curve, and the implementation of Embrace HES is no exception. Healthcare professionals will need to invest time in training to master the dual-injection process and understand the device’s behavior in varying clinical contexts. The educational phase is often full of overwhelming details, as practitioners must adjust to:
- The simultaneous injection technique for the liquid precursors
- Monitoring in real time to ensure proper polymerization
- Modifying standard operating procedures to adapt to the new device
- Communicating effectively within multidisciplinary teams to coordinate patient care
While these training requirements might seem intimidating or off-putting at first, they are key to unlocking the full potential of the device. In practice, most interventional radiologists are well-equipped to figure a path through these nerve-racking educational sessions. As more training programs and resources become available, we can anticipate smoother integration of the Embrace HES system into routine clinical workflow.
Improving Patient Outcomes: A Critical Look at Benefits and Limitations
From the patient’s perspective, the promise of a minimally invasive procedure with fewer complications is incredibly appealing. The high technical success rate and strong safety profile reported in the clinical trial suggest that Embrace HES could usher in clearer improvements in patient outcomes for those suffering from hypervascular tumors. Patients who previously faced a series of intimidating treatment options could soon have access to a method that reduces the chance of non-target embolization, minimizes imaging complications, and aligns more closely with targeted therapeutic goals.
However, it remains important to bear in mind that long-term data are still required. As with any innovative technique, continuing clinical studies and post-market evaluations will be crucial in establishing a more detailed picture of the therapy’s long-term benefits and limitations. Some of the open questions include:
- How does the system perform in varied anatomical scenarios over extended follow-up periods?
- Are there any delayed adverse events or unforeseen complications related to the hydrogel’s in vivo behavior?
- Can the device’s use be optimized across different subpopulations with hypervascular tumors?
These questions underscore that, while the preliminary results are super important and promising, ongoing research remains key to ensuring that such a groundbreaking device continues to deliver on its early promise without generating additional complications down the line.
Market Perspectives: Embracing Innovation Amid Regulatory Scrutiny
Beyond the clinical implications, the approval of the Embrace HES system sends a strong message to the medical device market. Investors, healthcare providers, and regulatory agencies alike are watching closely as the device begins its journey in real-world settings. The market for embolic agents has long been an arena marked by tricky parts and competitive pressures, where timing and innovation must align perfectly to yield success.
Several market considerations are worth noting:
| Factor | Implications |
|---|---|
| Regulatory Milestones | Boosts investor confidence and marks a new era for precision embolic devices. |
| Clinical Adoption | Requires extensive clinician training and real-world outcome verification. |
| Competitive Landscape | Challenges traditional embolization methods and pushes for continuous innovation. |
| Long-Term Efficacy | Ongoing studies and post-market surveillance will validate the anticipated benefits. |
These factors illustrate that while the arrival of such an innovative device is a major step forward, the road ahead is still full of puzzling bits and hidden complexities pertaining to market dynamics, regulatory follow-up, and patient outcome data collection. The device must continue to prove itself in a competitive landscape that is as dynamic as the clinical environment it is meant to serve.
Integrating Advanced Embolic Systems into Routine Practice: Challenges and Opportunities
The integration of any new medical technology into routine clinical practice is a matter of balancing potential benefits with the practical journey of daily clinical operations. For Embrace HES, opportunities abound—from its unique design to the clear advantages it holds over older embolic systems. Yet, there are also several hurdles that require careful planning and adjustment:
- Staff Education: Developing training modules that allow clinicians to poke around and master the dual-injection process.
- Workflow Adaptation: Adjusting scheduling and procedural steps to accommodate the new device’s operational specifics.
- Multi-disciplinary Coordination: Ensuring that radiologists, surgeons, and support staff are aligned to maximize patient care benefits.
- Ongoing Research: Continuing clinical trials and data collection to address any lingering uncertainties.
By managing these issues effectively, healthcare providers can make their way toward a smoother adoption process, paving the way for better patient outcomes and more efficient therapeutic protocols. The readiness of a clinical team to take the wheel and steer through these learning curves can ultimately determine the success of such initiatives at the bedside.
The Intersection of Clinical Innovation and Digital Transparency
While the promise of advanced liquid embolic technology energizes the clinical community, another dimension has become equally significant in today’s medical discourse: digital transparency in content delivery. Health organizations, especially those disseminating cutting-edge news like the Embrace HES approval, invest substantial effort in crafting detailed data privacy statements and cookie consent interfaces.
These detailed declarations of consent, such as those seen on platforms like BioSpace, serve dual purposes. They not only protect user data but also contribute to building public trust by explaining exactly how every single piece of web data is collected and used. For anyone trying to get around complicated bits of digital marketing and user tracking, such disclosures are a breath of fresh air—reinforcing the credibility of the content and the information provider.
It is essential for readers and industry stakeholders to appreciate the symbiotic relationship between clinical innovation and data privacy. On one hand, breakthrough medical devices promise to revolutionize treatment; on the other, transparency about online data practices upholds the integrity of health communications in an increasingly connected digital world.
The Future Outlook: Embracing Continuous Innovation in Medical Devices
Looking ahead, the approval of the Embrace Hydrogel Embolic System may very well represent a turning point in how hypervascular tumors are managed. As the interventional radiology community gets more comfortable with the system’s deployment, we can expect further refinements, enhancements, and perhaps even broader indications for its use.
Key aspects that lend optimism for the future include:
- Enhanced Clinical Outcomes: With ongoing training and a deepening body of clinical data, the promise of improved patient outcomes seems within reach.
- Evolving Device Design: Continuous iterations on the system’s design may help overcome any lingering technical issues and further minimize the risk of non-target embolization.
- Expanded Applications: Success in hypervascular tumor treatment may open up new avenues for using similar hydrogel-based embolics in other vascular conditions.
- Integrative Research Models: Close partnerships between device manufacturers, clinicians, and academic institutions will be essential in identifying the optimal protocols for wide-scale implementation.
Innovation in medicine is rarely linear, and the pathway to routine clinical adoption is full of tangled issues and nerve-racking moments. However, the collective willingness of the community to work through these challenges offers substantial hope. By carefully evaluating the outcomes, consistently training staff, and maintaining a robust digital communication infrastructure, the broader medical field can continue to benefit from such groundbreaking advancements.
Conclusion: Balancing Innovation with Skepticism in a Rapidly Evolving Field
In conclusion, the FDA approval of the Embrace Hydrogel Embolic System stands as a pivotal moment in the ongoing story of innovation in interventional oncology. While the device offers a promising solution to some of the traditional challenges of treating hypervascular tumors, its journey to becoming a routine clinical tool will require careful management of the subtle parts and convoluted bits that naturally arise with any new technology.
Doctors and healthcare professionals are now tasked with making their way through a complex blend of advanced device features, rigorous clinical trial data, and the nitty-gritty of training protocols. Simultaneously, they must also appreciate the equally critical realm of digital transparency and data privacy—a sector that ensures the trust and engagement of an informed public.
This transformative moment in medical device innovation is not just about a new tool in the interventional radiologist’s repertoire—it is about setting a new standard for how advanced therapies are tested, approved, and communicated. As more robust data are collected and more practitioners gain experience with the system, we can expect a gradual reshaping of treatment paradigms for hypervascular tumors.
Ultimately, the lessons learned from the Embrace HES system’s development and approval process will likely inform future innovations in the field. The dual emphasis on precise, effective clinical treatment and transparent, secure data practices will serve as a model for integrating technology seamlessly into patient care while maintaining high ethical and operational standards.
As the medical field continues to advance at a rapid clip, the interplay of scientific breakthroughs and digital transparency will undoubtedly shape how treatment options are communicated, evaluated, and ultimately adopted in both clinical settings and public discourse. It is an exciting time—full of promise and possibility—as we all work together to figure a path through the innovative yet occasionally intimidating landscape of modern medicine.
Originally Post From https://www.biospace.com/press-releases/instylla-gains-u-s-fda-premarket-approval-for-embrace-hydrogel-embolic-system
Read more about this topic at
Guerbet’s Lipiojoint Liquid Embolic Granted FDA …
U.S. Food and Drug Administration (FDA) grants …